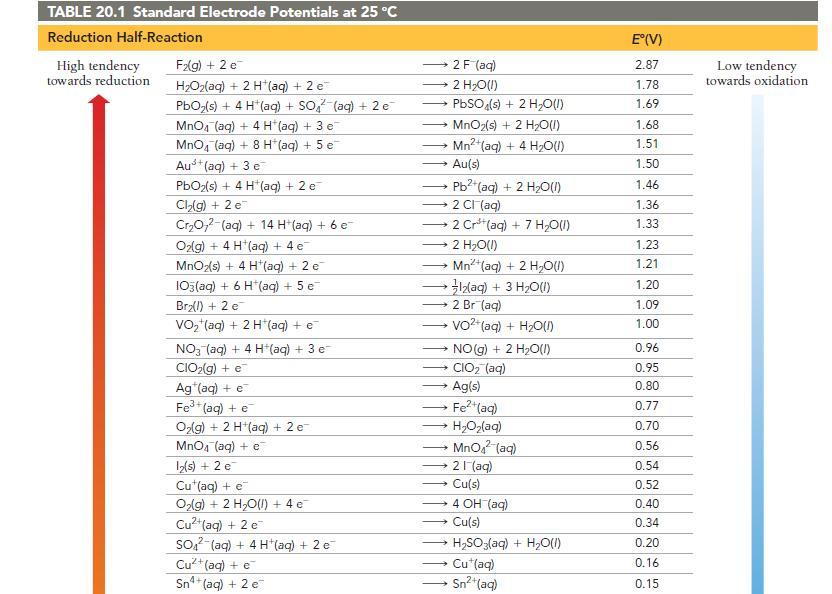
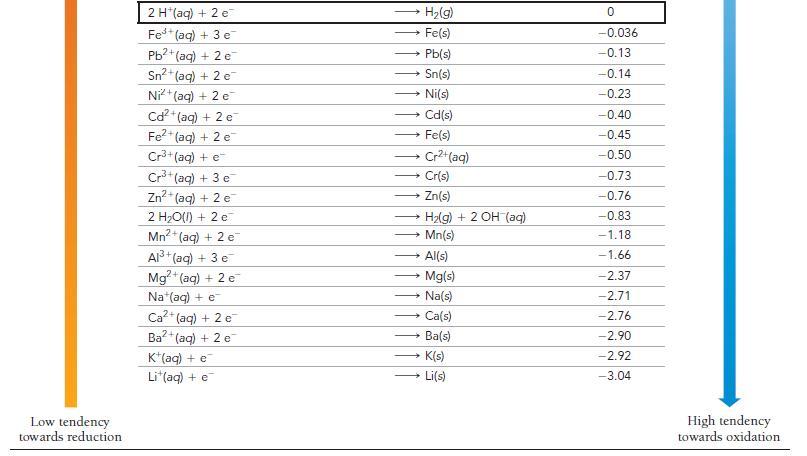
Use Table 20.1 to determine which statement is true of the voltaic cell pictured here. a) Sn
Question:
Use Table 20.1 to determine which statement is true of the voltaic cell pictured here.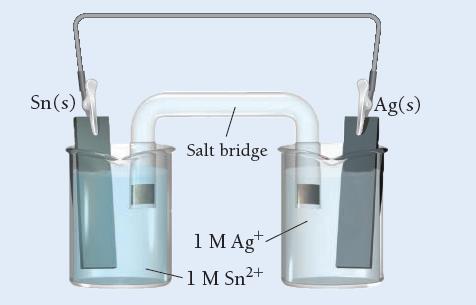
a) Sn is the anode; Ag is the cathode; electrons flow from left to right.
b) Sn is the cathode; Ag is the anode; electrons flow from left to right.
c) Sn is the anode; Ag is the cathode; electrons flow from right to left.
d) Sn is the cathode; Ag is the anode; electrons flow from right to left.
Transcribed Image Text:
Sn(s) Salt bridge + 1 M Ag 1 M Sn²+ Ag(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a Sn is the a...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose you are considering buying an apartment, your annual gross income is $82,000. You are allowed 35% of monthly gross income for PITI. Assume that your monthly consumption for living is $500,...
-
What is the value of E cel l for the voltaic cell pictured in Figure 19-10 and diagrammed as follows? Pt Fe+ (0.10 M), Fe+ (0.20 M)|| Agt (1.0M) Ag(s) Pt wire Anode Fe2+ (0.10 M) Fe3+ (0.20 M) 0.011...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Given the observed yields below, what is the 1-year forward rate, 4 years from now? [Hint: This is the 1-year return that will take you from the 4-year average annualized return (yield) to the 5-year...
-
Momence Associates is evaluating the performance of three divisions: Maple, Oaks, and Juniper. Using the following data, compute the return on investment and residual income for each division,...
-
Why must todays managers understand and utilize various motivational strategies?
-
Tyler Company reported the following costs on its financial statements (in thousands): REQUIRED: Using the reserve disclosure for Tyler Company in problem 13 and the data presented in this problem,...
-
Hiland Inc. manufactures snowsuits. Hiland is considering purchasing a new sewing machine at a cost of $2.45 million. Its existing machine was purchased five years ago at a price of $1.8 million; six...
-
The threshold frequency v0 describes the smallest light frequency capable of ejecting electrons from a metal. Determine the minimum energy E0 of a photon capable of ejecting electrons from a metal...
-
An electrode has a negative electrode potential. Which statement is correct regarding the potential energy of an electron at this electrode? (a) An electron at this electrode has a lower potential...
-
Balance the redox equation: Fe 2+ (aq) + MnO 4 (aq) GENERAL PROCEDURE Fe 3+ (aq) + Mn 2+ (aq)
-
Solve each problem. The graph of y = (x) represents the amount of water in thousands of gallons remaining in a swimming pool after x days. Water in a Swimming Pool (a) Estimate the initial and final...
-
Suppose Garageband.com has a 28% cost of equity capital and a 10% cost of debt capital. The firm's debt-to-equity ratio is 1.5. Garageband is interested in investing in a telecomm project that will...
-
In a particular population of moths, the frequency of the DD genotype (dark) occurring in the population is 0.04. What is the frequency of the dd genotype (light) occurring in the population?
-
Using the event budget information listed below, determine the total ticket sales you need to break even while also earning the desired profit of $1,912? Revenue Registration fee: $130 per person...
-
Use the passbook below to answer the question. Account 0000000004 Balance $389.00 Narne Ken Ritter Date Deposit Withdrawal Interest 1/02/06 $389.00 2/05./06 3/0206 $122.33 402.06 4/15.06 5/06.06...
-
Elizabeth determined that her tax liability was $3,954. Her employer withheld $3,476 from her paychecks during the year. Elizabeth's tax return would show: A. tax due of $3,954. B. refund of $3,476....
-
1. What are some important statements concerning the actual and potential impact of the computer and information technologies on society? Why 2. What are important statements concerning the actual...
-
Big Jim Company sponsored a picnic for employees and purchased a propane grill equipped with a standard-sized propane tank for the picnic. To make sure there was enough propane for all the cooking...
-
A child of mass 35 kg stands at the edge of a merry-go-round of mass 140 kg and radius 2.5 m, and both are initially at rest. The child then walks along the edge of the merry-goround until she...
-
Consider the yo-yo in Figure 9.8. What is the direction of the angular momentum vector of the yo-yo? Figure 9.8 ? M R V; = 0 W; = 0 INITIAL POSITION Uf = ? Wf = ? FINAL POSITION
-
Consider the rotational motion of the wheels of a car when the car is in motion. Use a drawing to show the direction of the angular momentum of each wheel.
-
The world has developed from smaller regions of trade into one Global trade region. Based on your work experience and what you have learned so far obtaining your certification, discuss how advances...
-
The accompanying video illustrates 10 social media fails in 2011. Students are to select two (2) incidents from the list and explain how they would have shaped the selected programs for a different,...
-
Based on your research, provide one example of robotics being used by a company (don't use an example from the readings). Include a discussion of how it affects operations. This post should be...

Study smarter with the SolutionInn App


