When N 2 O 5 (g) is heated, it dissociates into N 2 O 3 (g) and
Question:
When N2O5(g) is heated, it dissociates into N2O3(g) and O2(g) according to the reaction: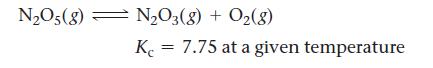
The N2O3(g) dissociates to give N2O(g) and O2(g) according the reaction: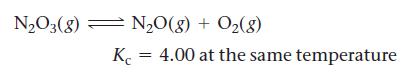
When 4.00 mol of N2O5(g) is heated in a 1.00-L reaction vessel to this temperature, the concentration of O2(g) at equilibrium is 4.50 mol/L. Find the concentrations of all the other species in the equilibrium system.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





