For the following, mix equal volumes of one solution from Group I with one solution from Group
Question:
For the following, mix equal volumes of one solution from Group I with one solution from Group II to achieve the indicated pH. Calculate the pH of each solution.
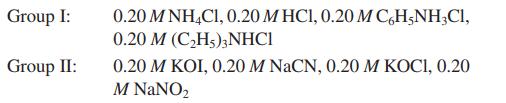
a. The solution with the lowest pH
b. The solution with the highest pH
c. The solution with the pH closest to 7.00
Transcribed Image Text:
Group I: Group II: 0.20 M NH4C1, 0.20 M HC1, 0.20 M C6H5NH₂Cl, 0.20 M (C₂H5)3NHC1 0.20 M KOI, 0.20 M NaCN, 0.20 M KOCI, 0.20 M NaNO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a The solution with the lowest pH The solution with the lowest pH will be the one formed by mixing NH4Cl from Group I with HCl from Group II When NH4C...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
What error in this program needs to be fixed? def sum(num1, num2): return num1 + num2 5.5, 6)) print(sum(4,
-
Consider the pooled t variable Tp from part (b) of the previous exercise. a. Use this t variable to obtain a pooled t confidence interval formula for 1 2 . b. The article Effect of Welding on a...
-
If there was any ambiguity on the application, should it be resolved in favor of the insured or the insurer? Provident Insurance, Inc., issued an insurance policy to a company providing an employee,...
-
A resistor and a capacitor are connected in series to an emf source. The time constant for the circuit is 0.870 s. (a) A second capacitor, identical to the first, is added in series. What is the time...
-
One critical-thinking skill is a heightened awareness of the danger of reaching a conclusion prior to acquiring missing information that were it known would have a reasonable probability of altering...
-
(EPS: Preferred Dividends, Options, and Convertible Debt) Earnings per share (EPS) is the most featured, single financial statistic about modern corporations. Daily published quotations of stock...
-
1. There are many market disruptions in the news today. (If you get stuck, on the last slide of the PowerPoint, I've given you 2 examples.) Choose any disruptor in the news and, (a) summarize the...
-
Mark Decker has identified four stocks for his portfolio, and he wants to determine the percentage of his total available funds he should invest in each stock. The alternative stocks include an...
-
A 0.100-g sample of the weak acid HA (molar mass = 100.0 g/mol) is dissolved in 500.0 g water. The freezing point of the resulting solution is -0.0056C. Calculate the value of K a for this acid....
-
Calculate the pH of a 0.10-M solution of sodium phosphate. (See Exercise 183.) Data in Exercise 183 Consider the species PO 4 3- , HPO 4 2- , and H 2 PO 4 - . Each ion can act as a base in water....
-
One of the underlying assumptions of the accounting model is the going concern assumption. When this assumption is questionable, valuation methods used for assets and liabilities may differ from...
-
State the amount of allowable deductions from gross estate in each of the following: EXERCISES/PROBLEMS A. State the amount of allowable deductions from the gross estate in each of the following 1....
-
Consider that company X issued convertible bonds with a nominal value of EUR 1000, currently quoted in the market at EUR 950, which may be converted in 100 common shares at any time after issue. The...
-
In order to send your first child to Law School when the time comes, you want to accumulate RM45,000 at the end of 17 years. Assuming that your savings account will pay 6.5% compounded annually,...
-
) REAL DATA: NASA only allows pilots between the heights of157.5 cm and 190.5 cm (62 to 75 inches). Its estimated (McDowellet al. 2008) that heights for self-identified US females is normally...
-
edro executed on January 1, 2016, a long-term loan from PRTC Bank in the amount of P6,000,000 payable within 10 years, with an annual interest of 2%. However, on January 31, 2020, the loan was...
-
Refer to Matthias Medical's financial statements, presented in Exercise 12-8. Required Calculate the following profitability ratios for 2013. a. Gross margin percentage b. Return on assets c. Return...
-
An investor sells a European call on a share for $4. The stock price is $47 and the strike price is $50. Under what circumstances does the investor make a profit? Under what circumstances will the...
-
Diethyl malonate (the starting material for the malonic ester synthesis) reacts with bromine in acid-catalyzed conditions to form a product with molecular formula C 7 H 11 BrO 4 . (a) Draw the...
-
Cinnamaldehyde is one of the primary constituents of cinnamon oil and contributes significantly to the odor of cinnamon. Starting with benzaldehyde and using any other necessary reagents, show how...
-
Draw the condensation product that is expected when each of the following esters is treated with sodium ethoxide followed by acid workup. (a) (b) (c) OEt OEt
-
Complete the income staComplete the balance sheet as of 1/31. Enter contra account amounts as negative numbers. TIP: You can leave the field blank if the balance in the account was $0tement for...
-
When calculating the balance of the Service Revenue account, you find that the balance is lower than expected. However, according to your Statement of Financial Position and your analysis of the...
-
Information for Computing the Allocation of Hospital Administration to Patient Care Service Departments Operating Departments Description Hospital Administration Custodial Services Laboratory Patient...

Study smarter with the SolutionInn App


