A cell is constructed using the following half-reactions: (a) What reactions should be observed at the anode
Question:
A cell is constructed using the following half-reactions: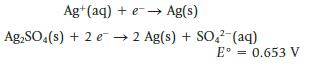
(a) What reactions should be observed at the anode and cathode?
(b) Calculate the solubility product constant, Ksp, for Ag2SO4.
Transcribed Image Text:
Ag+ (aq) + e → Ag(s) Ag₂SO4(s) + 2 e 2 Ag(s) + SO4²- (aq) E° = 0.653 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a The halfreaction with the more positive standard potential will occur at the cathode Therefore the ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A voltaic cell is constructed using the reaction (a) Write equations for the oxidation and reduction half-reactions. (b) Which half-reaction occurs in the anode compartment, and which occurs in the...
-
A potential of 0.142 V is recorded (under standard conditions) for a voltaic cell constructed using the following half reactions: (a) What is the standard reduction potential for the anode reaction?...
-
A voltaic cell is constructed using the reaction of chromium metal and iron(II) ions. Complete the following sentences: Electrons in the external circuit flow from the _______ electrode to the...
-
The accounting records of Shinault Inc. show the following data for 2017 (its first year of operations). 1. Life insurance expense on officers was $9,000. 2. Equipment was acquired in early January...
-
Dayna Moore, CEO of Layton Transmissions, sat dejected in her chair after reviewing the 2011 first-quarter financial reports on one of the companys core products: a standard, five-speed transmission...
-
Why are mergers reviewable under the Competition Act?
-
The 10-mm-diameter shank of the steel bolt has a bronze sleeve bonded to it. The outer diameter of this sleeve is 20 mm. If the yield stress for the steel is (y) st = 640 MPa, and for the bronze (y)...
-
The employees of Carson Bakery Company earn total wages of $7,780 during January. The total amount is taxable under FICA, FUTA, and SUTA. The state contribution rate for the company is 4.3%. The...
-
Respond using research from Journal Articles to validate your response. Discuss the laws that govern wages, pensions, and employee compensation in the USA.
-
Which of the following reactions is (are) product-favored at equilibrium? (a) Zn(s) + 1(s) Zn+ (aq) + 2 I (aq) (b) 2 Cl (aq) + (s) Cl(g) + 2 [ (aq) (c) 2 Nat (aq) + 2 Cl (aq) 2 Na(s) + Cl(g) (d) 2...
-
You want to set up a series of voltaic cells with specific cell potentials. A Zn 2+ (aq, 1.0 M) | Zn(s) half-cell is in one compartment. Identify several half-cells that you could use so that the...
-
What translation method is recommended in the CICA Handbook for self-sustaining foreign operations?
-
When consumers receive the Loan Estimate from a lender, what four main tasks they should perform on page one?
-
What are the 3 subscription levels on offer for QuickBooks Online Payroll? Explain.
-
Which assumption permits businesses to record property and equipment as assets at their cost without having to be concerned about what they are worth in case of liquidation in the near future?
-
Your property has six units: Unit # 1 rents for $950 per month and has a scheduled increase in month seven to $1,050 Unit # 2 rents for $1,100 per month Unit # 3 rents for $1,200 per month Units # 4...
-
What questions come from The Coahuila y Texas Immigration Law of 1825 raise?
-
Obesity among children is quickly becoming an epidemic across North America. Television and video games are part of the problem. To gauge to what extent nonparticipation in organized sports...
-
On 1 July 2021, Croydon Ltd leased ten excavators for five years from Machines4U Ltd. The excavators are expected to have an economic life of 6 years, after which time they will have an expected...
-
A Mealy sequential circuit with four output variables is realized using a 22V10. What is the maximum number of input variables it can have? What is the maximum number of states? Can any Mealy circuit...
-
Show how the left shift register of Figure 2-41 could be implemented using a CPLD. Draw a diagram. Give the equations for the flip-flop D inputs. always @ (posedge CLK) begin if (CLR) else if (Ld)...
-
An N-bit bidirectional shift register has N parallel data inputs, N outputs, a left serial input (LSI), a right serial input (RSI), a clock input, and the following control signals: Load: Load the...
-
Markus recently lost his house and contents in a bushfire that he constructed 5 years ago for $180,000 and spent $40,000 at that time on various appliances, furniture, fixtures and fittings for the...
-
A stock has an expected return of 0.16, its beta is 0.63, and the expected return on the market is 0.1. What must the risk-free rate be? You own a portfolio equally invested in a risk-free asset and...
-
Vinny's Vineyard (VV) has a current market value of $100 million and a D/E ratio of 0.2. VV believes they can increase value by increasing leverage and they intend to double their current debt and...

Study smarter with the SolutionInn App


