A line in the Balmer series of emission lines of excited H atoms has a wavelength of
Question:
A line in the Balmer series of emission lines of excited H atoms has a wavelength of 410.2 nm (Figure 6.10). What color is the light emitted in this transition? What quantum levels are involved in this emission line? That is, what are the values of ninitial and nfinal?
Data given in Figure 6.10
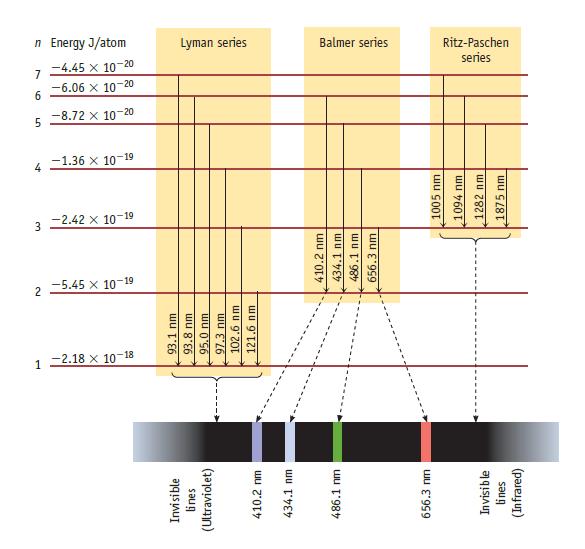
Transcribed Image Text:
Invisible lines (Ultraviolet) 410.2 nm 434.1 nm 486.1 nm 656.3 mm Invisible lines (Infrared) 12.18 x 10-18 93.1 nm 93.8 nm 95.0 nm 97.3 nm 102.6 nm 121.6 nm 2 -5.45 x 10-19 3-2.42 x 10-19 410.2 nm 434.1 nm 486.1 nm 656.3 nm 4 -1.36 x 10-19 1005 nm 1094 nm 1282 nm. 1875 nm 5 -8.72 x 10-20 9 -6.06 x 10-20 7-4.45 x 10-20 n Energy J/atom Lyman series Balmer series series Ritz-Paschen
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To determine the color of the light emitted in the Balmer series transition with a wavelength of 410...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Study the energy-level diagram shown in figure 18.20. The Balmer series of spectral lines all involve transitions to the n = 2 energy level, and the Lyman series in the ultraviolet involves...
-
Wilson Corporation is performing the test of impairment of its Technology reporting unit at the end of the year. Wilson has determined the fair value of the unit using a multiple of earnings approach...
-
Figure 37.7 identified the wavelengths of four lines in the Balmer series of hydrogen. a. Determine the Balmer formula n and m values for these wavelengths. b. Predict the wavelength of the fifth...
-
Estimate the errors involved in Exercise 63, parts (a) and (b). How large should be in each case to guarantee an error of less than 0.00001?
-
The advertising agency promoting the new Breem dishwashing detergent wants to get the best exposure possible for the product within the $100,000 advertising budget ceiling placed upon it. To do so,...
-
Pam Corporation owns a 40 percent interest in the outstanding common stock of Sun Corporation, having acquired its interest for $2,400,000 on January 1, 2016, when Sun's stockholders' equity was...
-
Predicting Delayed Flights. The file FlightDelays . jmp contains information on all commercial flights departing the Washington, DC area and arriving at New York during January 2004. For each flight...
-
Duval Manufacturing recently reported the following information: Net income $600,000 ROA 8% Interest expense $225,000 Duvals tax rate is 35%. What is its basic earning power (BEP)?
-
MyBnB started a home rental company on January 1. As of November 30, MyBnB reported the following balances. The company does not yet have a balance in Retained Earnings because this is its first year...
-
A table wine has a pH of 3.40. What is the hydronium ion concentration of the wine? Is it acidic or basic?
-
Looking at Figure 1-8, in what years would you have chosen to visit the Canadian Rockies rather than Washington, D.C.? 1971 1973 1975 1977 1979 1981 1983 1985 1987 1989 1991 1993 1995 1997 1999 2001...
-
An element on the surface of a drive shaft is in pure shear and is subjected to stresses xy = 2700 psi, as shown in the figure. Using Mohrs circle, determine the following. (a) The stresses acting...
-
Compliance With Payroll Reporting Applying Statutory Requirements Describe how the organisation has applied statutory requirements for the organisation's payroll reporting through Single Touch...
-
Kate is 31 years old, has two dependent children, and files Head of Household. What is the amount that her 2022 California gross income must exceed for her to be required to file a tax return?
-
where do you assess for splenic enlargement?
-
How does globalization influence world music, particularly in terms of cross-cultural exchange and hybridization, leading to the enrichment of musical traditions? Furthermore, how do these processes...
-
How might a company attempt to make its financials appear worse than they are?
-
Describe the operations of the NASDAQ market. Compare it with an exchange, such as the NYSE.
-
Refrigerant-134a enters an adiabatic compressor as saturated vapor at 120 kPa at a rate of 0.3 m3/min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 80 percent,...
-
In this chapter, we explained why pyrrole is such a weak base, but we did not discuss the acidity of pyrrole. In fact, pyrrole is 20 orders of magnitude more acidic than most simple amines. Draw the...
-
Rimantadine is an antiviral drug used to treat people infected with life-threatening influenza viruses. Identify the starting ketone that would be necessary in order to prepare rimantadine via a...
-
Benzphetamine is an appetite suppressant that is marketed under the trade name Didrex and used in the treatment of obesity. Identify at least two different ways to make benzphetamine via a reductive...
-
What novel insights can be gleaned from the burgeoning field of positive psychology regarding the cultivation of psychological strengths and virtues as a means of buffering against the detrimental...
-
Tektron Industries applies overhead on the basis of 200% of direct labor cost. Job No. 275 is charged with $30,000 of direct materials costs and $40,000 of manufacturing overhead. What are the total...
-
QUESTION 1 Given the following information: Units produced Materials (kg) Material Costs Direct Labour (Hours) Original Budget 1,000 units 400 kg $8,000 Actual 1,070 units 409 kg See other info...

Study smarter with the SolutionInn App


