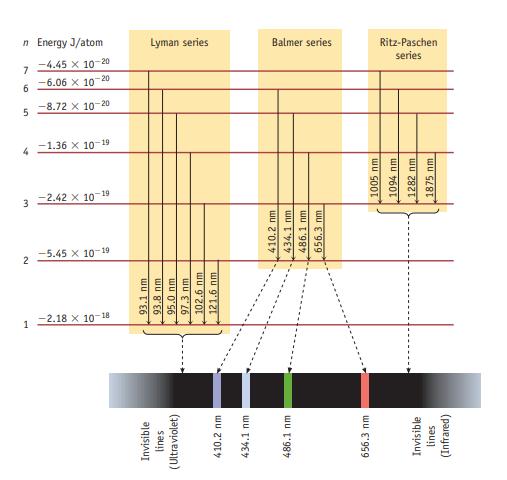
A photon with a wavelength of 93.8 nm strikes a hydrogen atom, and light is emitted by
Question:
A photon with a wavelength of 93.8 nm strikes a hydrogen atom, and light is emitted by the atom. How many emission lines would be observed? At what wavelengths? Explain briefly (see Figure 6.10).
Data given in figure 6.10
Transcribed Image Text:
Invisible lines (Ultraviolet) 410.2 nm 434.1 mm 486.1 mm 656.3 mm Invisible lines (Infrared) 1 -2.18 X 10-18 93.1 nm 93.8 nm 95.0 nm 97.3 nm 102.6 nm 121.6 nm N -5.45 x 10-19 3 -2.42 x 10-19 410.2 mm 434.1 nm. 486,1 nm 656.3 nm 1005 nm 1094 nm 1282 nm 1875 nm 7 -1.36 x 10-19 5 -8.72 x 10-20 -6.06 x 10-20 -4.45 x 10-20 n Energy J/atom 9 7 Lyman series Balmer series series Ritz-Paschen
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The number of emission lines that would be observed depends on the energy levels of the hydrogen ato...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Canadian Steel: Role of Information Systems (IS) Canadian Steel is the 5th largest integrated steel manufacturer in North America and the largest in Canada. Headquartered in Mitchell, Ontario, it can...
-
Consider only transitions involving the n = 1 through n = 4 energy levels for the hydrogen atom (see Figures 6.7 and 6.10). (a) How many emission lines are possible, considering only the four quantum...
-
Consider only transitions involving the n = 1 through n = 5 energy levels for the H atom (see Figures 6.7 and 6.10). (a) How many emission lines are possible, considering only the five quantum...
-
Read the Poem Little Birds Flying and answer the following questions: What it is notifying? To whom it is notifying? How is the work two dimensional? What does it take to realize the project? ...
-
Refer to Problem M4-8. There is a saddle point in this game, making it a pure strategy game. Ignore this and solve it as a mixed strategy game. What special condition in the solution indicates that...
-
The information that follows relates to equipment owned by Gaurav Limited at December 31, 2017: Cost ...................................................................................... $10,000,000...
-
What is a split-ballot technique and what is it used for?
-
Accounting Today identified the top accounting firms in 10 geographic regions across the United States. All 10 regions reported growth in 2013. the Southeast and Gulf Coast regions reported growth of...
-
4. Consider the following program: COUNTER(c) 1: xc. 2: y+0. 3: while (20) do 4: 2-2-1. You may assume that c is a non-negative integer. 5: y+y+1. 6: return y. What value does COUNTER(c) return?...
-
In principle, which of the following can be determined? (a) The energy of an electron in the H atom with high precision and accuracy (b) The position of a high-speed electron with high precision and...
-
Which of these are observable? (a) Position of an electron in an H atom (b) Frequency of radiation emitted by H atoms (c) Path of an electron in an H atom (d) Wave motion of electrons (e) Diffraction...
-
What is the purpose of Form 940? How often is it prepared, and what is the due date?
-
In one of the readings, Collins lists "Ten Lessons I Learned from Peter Drucker." This was his Foreward to the 50th Anniversary Edition of Drucker's still highly praised, widely read and influential...
-
1. Nykke, Inc., is considering establishing a subsidiary in Canada that would manufacture and sell sports shoes locally. The project would require an initial investment of 50 million Canadian dollars...
-
Here you are asked to think about what you have discovered about your self and interpersonal communication in relationships in the final weeks of this very challenging semester. What have you...
-
What elements have to change to allowa distributive bargaining situation to become more integrative? Discuss how you would apply the integrative negotiation steps to make a used car distributive...
-
What financial skills should Sabatino have to help Forcefield Energy achieve its stated goals?
-
For each pair of funds listed below, select the fund that would be less risky and briefly explain your answer. a. Growth versus growth-and-income b. Equity-income versus high-grade corporate bonds c....
-
Determine which of the following limits exist. Compute the limits that exist. lim x-0 1- + 3x X
-
Why is it not necessary to know absolute half-cell potentials to determine the emf of an electrochemical cell?
-
What is the voltage between the terminals of a battery in which the contents are in chemical equilibrium?
-
By convention, the anode of a battery is where oxidation takes place. Is this true when the battery is charged, discharged, or both?
-
Tax Optimization Mastery This activity will provide practical insights into tax optimization, demonstrating the importance of the 6 2 5 1 form for individuals with specific income streams and...
-
Illinois Company prepared the following bank reconciliation at May 3 1 :Balance per bankAdditions:$ 1 , 2 9 5 Deposits in transitCheck incorrectly charged to our bank balanceDeductions: 2 5 8 1 0 2...
-
Prepare journal entries for the following transaction using the accrual basis of accounting. Received $2,000 from a charge client for services performed last month. For the toolbar, press ALT+F10...

Study smarter with the SolutionInn App


