(a) Write a balanced chemical equation for the formation of 1 mol of MgO(s) from the elements...
Question:
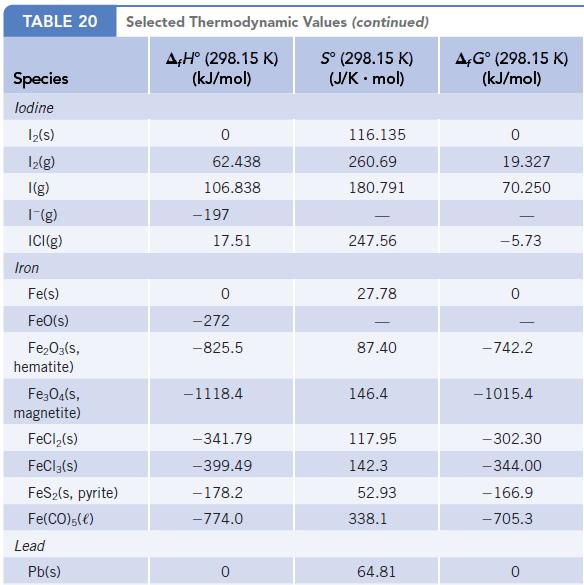
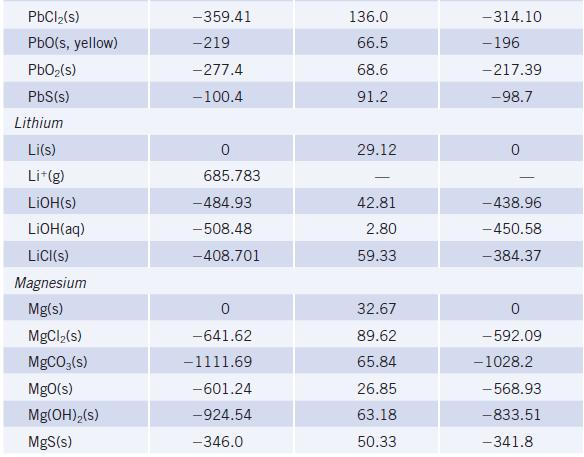
(a) Write a balanced chemical equation for the formation of 1 mol of MgO(s) from the elements in their standard states. (Find the value for ∆fH° for MgO(s) in Appendix L.)
(b) What is the standard enthalpy change for the reaction of 2.5 mol of Mg with oxygen?
Data given in Appendix L
Transcribed Image Text:
TABLE 20 Species lodine 1₂(s) 1₂(g) I(g) 1-(g) ICI(g) Iron Fe(s) FeO(s) Fe₂O3(s, hematite) Fe3O4(s, magnetite) FeCl₂(s) FeCl3(s) FeS₂(s, pyrite) Fe(CO)5(e) Lead Pb(s) Selected Thermodynamic A,Hº (298.15 K) (kJ/mol) 0 62.438 106.838 - 197 17.51 0 -272 -825.5 -1118.4 -341.79 -399.49 -178.2 -774.0 0 Values (continued) Sº (298.15 K) (J/K . mol) 116.135 260.69 180.791 247.56 27.78 87.40 146.4 117.95 142.3 52.93 338.1 64.81 A,Gº (298.15 K) (kJ/mol) 0 19.327 70.250 -5.73 -742.2 -1015.4 -302.30 -344.00 - 166.9 -705.3 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The balanced chemical equation for the formation of 1 mol of M...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
(a) Write a balanced chemical equation for the formation of 1 mol of Cr 2 O 3 (s) from Cr and O 2 in their standard states. (Find the value for f H for Cr 2 O 3 (s) in Appendix L.) (b) What is the...
-
For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and use Appendix C to...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
An important U.S. government organization charged with setting human resource management guidelines is O the EEOC (Equal Employment Opportunity Commission). the OSHA (Occupational Safety and Health...
-
Discuss the following statement: Union security means job security for union members.
-
The owner of Colonial Adventure Tours knows the importance of the SQL language in database management. He realizes that he can use SQL to perform the same functions that you performed with queries in...
-
The Wide World of Fluids article titled "10 Tons on 8 psi,". A massive, precisely machined, 6-ft-diameter granite sphere rests on a 4-ft-diameter cylindrical pedestal as shown in Fig. P6.75. When the...
-
Use the Codification to identify accounting authority (location within the Codification) governing each of the following: a. The accounting for prepaid advertising b. The accounting for...
-
What increases brain activity and how does it relate to what is consciousness? What leads to the loss of consciousness? illustrate the process of consciousness? is consciousness gradual? Explain. How...
-
Using spdf and noble gas notations, write electron configurations for atoms of the following elements. (Try to do this by looking at the periodic table but not at Table 7.3.) (a) Strontium, Sr. This...
-
The half-cells Sn 2+ (aq) | Sn(s) and Cl 2 (g) | Cl (aq) are linked to create a voltaic cell. (a) Write equations for the oxidation and reduction half-reactions and for the overall (cell) reaction....
-
Suppose that labor market experience is the only characteristic that influences wages. Suppose also that males and females have different levels of experience and have different "wage functions"...
-
I A car bounces up and down on its springs at \(1.0 \mathrm{~Hz}\) with only the driver in the car. Now the driver is joined by four friends. The new frequency of oscillation when the car bounces on...
-
A coyote can locate a sound source with good accuracy by comparing the arrival times of a sound wave at its two ears. Suppose a coyote is listening to a bird whistling at \(1000 \mathrm{~Hz}\). The...
-
All other things being equal, species that inhabit cold climates tend to be larger than related species that inhabit hot climates. For instance, the Alaskan hare is the largest North American hare,...
-
A heavy brass ball is used to make a pendulum with a period of \(5.5 \mathrm{~s}\). How long is the cable that connects the pendulum ball to the ceiling? A. \(4.7 \mathrm{~m}\) B. \(6.2 \mathrm{~m}\)...
-
Assume that the opening of the ear canal has a diameter of\(7.0 \mathrm{~mm}\). For this problem, you can ignore any focusing of energy into the opening by the pinna, the external folds of the ear....
-
The following facts pertain to the e-Commerce Web site for WCE-Comp, a personal computer manufacturer and seller. Privacy Statement a. All information collected on this Web site will be used only for...
-
For each equation, (a) Write it in slope-intercept form (b) Give the slope of the line (c) Give the y-intercept (d) Graph the line. 7x - 3y = 3
-
Determine the force in member AB, BC and BD which is used in conjunction with the beam to carry the 30-k load. The beam has a moment of inertia of I = 600 in 4 , the members AB and BC each have a...
-
The cantilevered beam AB is additionally supported using two tie rods. Determine the force in each of these rods. Neglect axial compression and shear in the beam. For the beam, I b = 200 (10 6 ) mm 4...
-
Determine the force in member GB of the truss. AE is constant. 10 ft A D B 10 ft 10 ft 10ft 10 ft 10 k 15 k 5 k
-
On the 1st of January, David by three separate letters which he posted to Ann, Bev and Cathy informed them that he was selling his 2004 BMW car for $1M. He stated in the letter that the offer would...
-
Write an equation for a rational function with: Vertical asymptotes at x = 1 and x = -3 x-intercepts at x= -6 and x = 6 y-intercept at 7
-
Q1. Jassim Compagny is producing only one product. Two types of direct materials are used to produce this product: direct material type( A) and direct material type B. The estimated data for Jassim...

Study smarter with the SolutionInn App


