Acrolein is used to make plastics. Suppose this compound can be prepared by inserting a carbon monoxide
Question:
Acrolein is used to make plastics. Suppose this compound can be prepared by inserting a carbon monoxide molecule into the C—H bond of ethylene.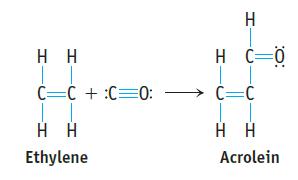
(a) Which is the stronger carbon–carbon bond in acrolein?
(b) Which is the longer carbon–carbon bond in acrolein?
(c) Is ethylene or acrolein polar?
(d) Use bond dissociation enthalpies to predict whether the reaction of CO with C2H4 to give acrolein is endothermic or exothermic.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





