Amino acids are an important group of compounds. At low pH, both the carboxylic acid group (CO
Question:
Amino acids are an important group of compounds. At low pH, both the carboxylic acid group (—CO2H) and the amine group (—NHR) are protonated. However, as the pH of the solution increases (say, by adding base), the carboxylic acid proton is removed, usually at a pH between 2 and 3. In a middle range of pHs, therefore, the amine group is protonated, but the carboxylic acid group has lost the proton. (This is called a zwitterion.) At more basic pH values, the amine proton is dissociated.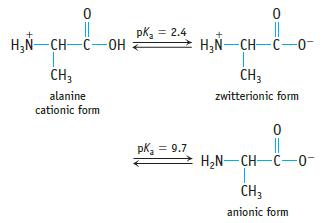
What is the pH of a 0.20 M solution of alanine hydrochloride, [NH3CHCH3CO2H]Cl?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





