An unknown solid acid is either citric acid or tartaric acid. To determine which acid you have,
Question:
An unknown solid acid is either citric acid or tartaric acid. To determine which acid you have, you titrate a sample of the solid with aqueous NaOH and from this determine the molar mass of the unknown acid. The appropriate equations are as follows: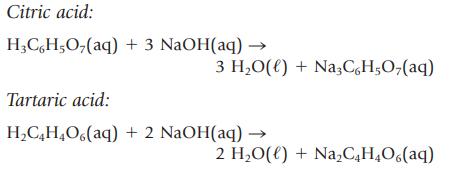
Transcribed Image Text:
Citric acid: H3CH₂O₂(aq) + 3 NaOH(aq) - 3 H₂O(l) + Na3C,H,O,(aq) Tartaric acid: H₂C4H₂O(aq) + 2 NaOH(aq) → 2 H₂O(l) + Na₂C4H₂O6(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine which acid citric acid or tartaric acid you have you can titrate a sample of the solid with aqueous NaOH and then determine the molar mas...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Evergreen company has assets of $500 million and $125 million in liabilities. For the past year, Evergreen earned $150 million and paid out $20 million in dividends. What is the company's return on...
-
Suppose that the stock price S, follows lognormal distribution St= Soe(a-8-0.50) t+oiz Prove that the conditional expectation of lognormal prices, when terminal stock price ST falls below Kis where d...
-
Assume analysts provide the following types of information. Assume no short selling and a risk free-rate of 10%. What is the optimal investment? Expected Return Standard Deviation Asset 1 10% 5%...
-
Why must we recognize and address challenges caused by diversity and work to implement a more inclusive healthcare workforce?
-
Compute the Cpk measure of process capability for the following machine and interpret the findings. What value would you have obtained with the Cp measure? Machine . USL = 100 LSL = 70 Process = 5...
-
Studies indicate that good leaders are typically positive and enthusiastic. In what ways do the tips cited in the vignette suggest the possession of these qualities by the various leaders who offer...
-
Chandrasekhar (1961) has shown that the above (Bnard) problem can be solved in terms of the vorticity, which reduces the problem to a sixth-order eigenvalue problem for the perturbation in...
-
Deleon Inc. is preparing its annual budgets for the year ending December 31, 2017. Accounting assistants furnish the data shown below. An accounting assistant has prepared the detailed manufacturing...
-
(20 pts.) Hadamard matrices. The Hadamard matrices Ho, H1, H2,... are defined as follows: Ho is the 11 matrix [1] For k > 0, Hk is the 2k 2k matrix So, for example, H = and [Hk-1 Hk-1 Hk = Hk-1 -Hk-1...
-
To analyze an iron-containing compound, you convert all the iron to Fe 2+ in aqueous solution and then titrate the solution with standardized KMnO 4 . The balanced, net ionic equation is A 0.598-g...
-
You have 0.954 g of an unknown acid, H 2 A, which reacts with NaOH according to the balanced equation If 36.04 mL of 0.509 M NaOH is required to titrate the acid to the second equivalence point, what...
-
Construct and assess alternative strategic options for Korres.
-
Complete the following chart by using Wien's law. You may use a calculator and the Wien's law formula, or the interactive graph Planck Law for Blackbodies to calculate temperature when given...
-
Use the following information to answer questions. Assets (in thousands)AmountDurationLiab&NWAmountDuration__ Cash$1000.001-yr CD$6001.00 C&ILoans$4001.256-yr CD$3005.00 Mortgageloans$5007.00NW...
-
c. Growth Company has existing debt issued three years ago with a coupon rate of 5.7%. The firm just issued new debt at par with a coupon rate of 6.9%. What is Growth Company's cost of debt? (Select...
-
A large architectural firm has just landed a contract to build a hospital. Seven architects currently work 40 hours per week in this firm, and all are available to work full-time on this project. The...
-
Josh and Nancy are at Coachella. They made plans to meet up after Flume's set, but for now, they're both watching Muse at the main stage, but from different locations. At Nancy's location the music...
-
Alex, Alicia, and Juan ll orders in a fast-food restaurant. Alex incorrectly fills 20% of the orders he takes. Alicia incorrectly fills 12% of the orders she takes. Juan incorrectly fills 5% of the...
-
Cornell and Roberts are partners who agree to admit Stanley to their partnership. Cornell has a capital balance of $80,000 and Roberts has a capital balance of $120,000. Cornell and Roberts share net...
-
Which of the following processes is spontaneous? a. The reversible isothermal expansion of an ideal gas. b. The vaporization of superheated water at 102C and 1 bar. c. The constant pressure melting...
-
One joule of work is done on a system, raising its temperature by one degree centigrade. Can this increase in temperature be harnessed to do one joule of work? Explain.
-
Your roommate decides to cool the kitchen by opening the refrigerator. Will this strategy work? Explain your reasoning.
-
Use the May 31 fiscal year-end information from the following ledger accounts (assume that all accounts have normal balances). General Ledger Retained Earnings Date May 31 PR Debit Account Number 318...
-
Wheels, Inc. manufactures wheels for bicycles, tricycles, and scooters. For each cost given below, determine if the cost is a product cost or a period cost. If the cost is a product cost, further...
-
The nose of an ultralight plane is pointed south, and its airspeed indicator shows 30 m/s. The plane is in a 16 m/s wind blowing toward the southwest relative to the earth. You may want to review...

Study smarter with the SolutionInn App


