Calculate the molar enthalpy of formation, f H, of solid lithium fluoride from the lattice energy
Question:
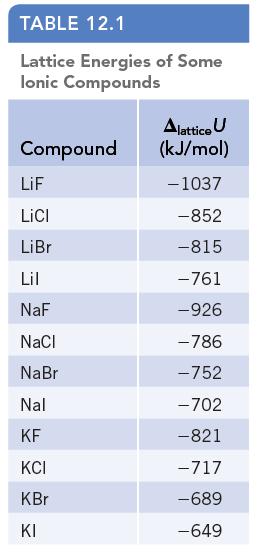
Calculate the molar enthalpy of formation, Δf H°, of solid lithium fluoride from the lattice energy (Table 12.1) and other thermochemical data. The enthalpy of formation of Li(g), Δf H° [Li(g)] =159.37 kJ/mol. Other required data can be found in Appendices F and L.
Data given in Table 12.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





