Commercial sodium hydrosulfite is 90.1% Na 2 S 2 O 4 . The sequence of reactions used
Question:
Commercial sodium “hydrosulfite” is 90.1% Na2S2O4. The sequence of reactions used to prepare the compound is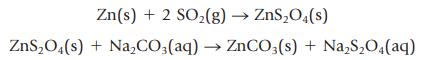
(a) What mass of pure Na2S2O4 can be prepared from 125 kg of Zn, 500. g of SO2, and an excess of Na2CO3?
(b) What mass of the commercial product would contain the Na2S2O4 produced using the amounts of reactants in part (a)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





