Compare the compounds in each set below and decide which is expected to have the higher entropy.
Question:
Compare the compounds in each set below and decide which is expected to have the higher entropy. Assume all are at the same temperature. Check your answers using data in Appendix L.
(a) HF(g), HCl(g), or HBr(g)
(b) NH4Cl(s) or NH4Cl(aq)
(c) C2H4(g) or N2(g) (two substances with the same molar mass)
(d) NaCl(s) or NaCl(g)
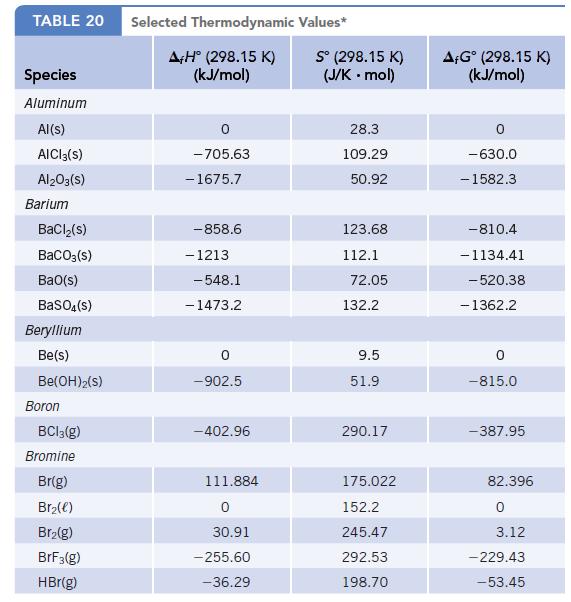
Data given in Appendix L
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





