Construct a rough plot of pH versus volume of base for the titration of 25.0 mL of
Question:
Construct a rough plot of pH versus volume of base for the titration of 25.0 mL of 0.050 M HCN with 0.075 M NaOH.
(a) What is the pH before any NaOH is added?
(b) What is the pH at the halfway point of the titration?
(c) What is the pH when 95% of the required NaOH has been added?
(d) What volume of base, in milliliters, is required to reach the equivalence point?
(e) What is the pH at the equivalence point?
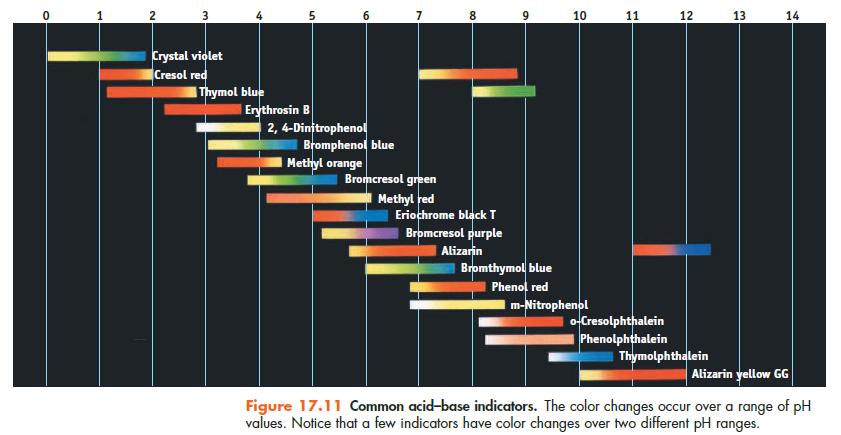
(f) What indicator would be most suitable for this titration? (Figure 17.11.)
(g) What is the pH when 105% of the required base has been added?
Data given in Figure 17.11
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:




