Dinitrogen trioxide, N 2 O 3 , has the structure shown here. The oxide is unstable, decomposing
Question:
Dinitrogen trioxide, N2O3, has the structure shown here.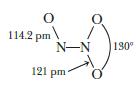
The oxide is unstable, decomposing to NO and NO2 in the gas phase at 25°C.![]()
(a) Draw resonance structures for N2O3 and explain why one N—O bond distance is 114.2 pm, whereas the other two bonds are longer (121 pm) and nearly equal to each other.
(b) For the decomposition reaction, ΔrH° =+40.5 kJ/mol and ΔrG° = −1.59 kJ/mol. Calculate ΔS° and K for the reaction at 298 K.
(c) Calculate ΔfH° for N2O3(g).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





