Equal amounts of two acidsHCl and HCO 2 H (formic acid)are placed in aqueous solution. When equilibrium
Question:
Equal amounts of two acids—HCl and HCO2H (formic acid)—are placed in aqueous solution. When equilibrium has been achieved, the HCl solution has a much greater electrical conductivity than the HCO2H solution. Which reaction is more product-favored at equilibrium? 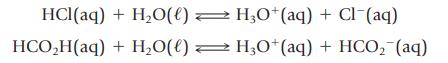
Transcribed Image Text:
HCl(aq) HCO₂H(aq) + H₂O(l) + H₂O(l) H3O+ (aq) + Cl (aq) H3O+ (aq) + HCO₂ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The difference in electrical conductivity between two solutions can be an indicator of th...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
At high temperatures, a dynamic equilibrium exists between carbon monoxide, carbon dioxide, and solid carbon. At 850oC, Kc is 0.153. a. What is the value of Kp? b. If the original reaction system...
-
Tesselek Technology, Inc., is a relatively new company and has been operating for only the past five years. It was set up by two leading scientists Mr. Tess and Mr. Lek. The company is involved in...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
(c) It is known that although variance is a widely used measure of investment risk, variance does not satisfy the concept of subadditivity. This means that a risk measure should reflect the fact that...
-
There is no point in the United States complaining about trade policies in Japan and Europe. Each country has a right to do whatever is in its own best interest. Instead of complaining about foreign...
-
N 2. (a) State and explain the four rules governing view factor relations in radiation. (8 marks) (b) In a shell-and-tube heat exchanger, what factors should be taken into consideration when deciding...
-
A hydraulic jack with a plunger of 30 mm diameter has a stroke of 100 mm, and a ram of 300 mm diameter. A lever arrangement with a leverage of 10 is used to drive the plunger. If a weight of 5 kN is...
-
A solvent-recovery plant consists of a plate-column absorber and a plate-column stripper. Ninety percent of the benzene (B) in the gas stream is recovered in the absorption column. Concentration of...
-
A telecommunications company owns satellites A, B, C and D, which are in orbit around the Earth. At a particular instant in time, all the satellites lie on a circle whose diameter crosses the centre...
-
Identify each of the following statements as either true or false. (a) At equilibrium the rates of the forward and reverse reactions are equal. (b) When a reaction reaches equilibrium the forward and...
-
A 0.20 mol sample of magnesium burns in air to form 0.20 mol of solid MgO. What amount (moles) of oxygen (O 2 ) is required for a complete reaction? P4(s) + 6 Cl(g) 4 PCL3 (1) reactants product
-
Assume a saver deposits $500 for two years in a bank account that pays 10 percent interest. What is the simple interest over two years? What would it be if the account were compounded annually? Why...
-
Atoms can be slowed to rest by shooting photons at them. We can think of the photons as simply being absorbed by the atom. Imagine you are an atomic physicist who wants to laser cool rubidium atoms...
-
Calculate the loan risk associated with a $3 million, five-year loan to a BBB-rated corporation in the computer parts industry that has a duration of 3.5 years. The cost of funds for the bank is 8...
-
Analyse the quality of the Java codes below public static boolean leap(int y) { String tmp = String.valueOf(y); if (tmp.charAt(2) == '1' || tmp.charAt(2) == '3' || tmp.charAt(2) == 5 || tmp.charAt(2)...
-
Calculate the loan risk associated with a $2 million, five-year loan to a BBB-rated corporation in the computer parts industry that has a duration of 6.4 years. The cost of funds for the bank is 8...
-
Kohl's Department Store and Target Corporation buy their clothing from Form Fitters Inc., a merchandising firm, that has budgeted its activity for December according to the following information: -...
-
It seems as if consolidated net income is always less than the sum of the parent's and subsidiary's separately calculated net incomes. Is it possible that the consolidated net income of the two...
-
You continue to work in the corporate office for a nationwide convenience store franchise that operates nearly 10,000 stores. The per- store daily customer count (i.e., the mean number of customers...
-
In each case below, draw the curved arrow(s) required in order to convert the first resonance structure into the second resonance structure. In each case, begin by drawing all lone pairs, and then...
-
Consider the isothermal expansion of 2.35 mol of an ideal gas at 415 K from an initial pressure of 18.0 bar to a final pressure of 1.75 bar. Describe the process that will result in the greatest...
-
An automobile tire contains air at 225. 10 3 Pa at 25.0C. The stem valve is removed and the air is allowed to expand adiabatically against the constant external pressure of one bar until P = P...
-
Why are there such discrepancies in the view of performance by the manager and the employees? Is this a good place for 360 feedback? How much should the manager be influenced by employee opinions?...
-
Discuss the impact of non-ideal mixtures on distillation column design, focusing on vapor-liquid equilibrium (VLE) modeling and the use of activity coefficient-based models like UNIQUAC and NRTL
-
How does extractive distillation differ from conventional distillation in terms of solvent selection, mechanism, and industrial applications? Provide examples where extractive distillation is...
Literature An Introduction To Reading And Writing Compact 4th Edition - ISBN: 0132233924 - Free Book

Study smarter with the SolutionInn App


