In Example 4.2, you found that a particular mixture of CO and H 2 could produce 407
Question:
In Example 4.2, you found that a particular mixture of CO and H2 could produce 407 g CH3OH.
![]()
If only 332 g of CH3OH is actually produced, what is the percent yield of the compound?
Data given in Example 4.2
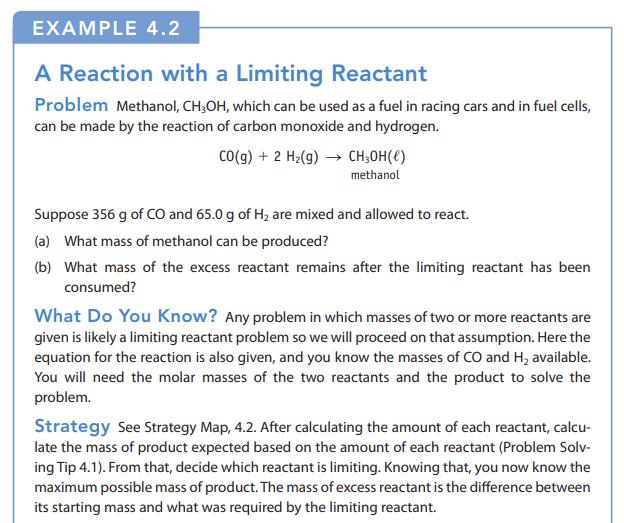
Transcribed Image Text:
CO(g) + 2 H₂(g) → CH₂OH()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the percent yield of CH3OH we can use the following equatio...View the full answer

Answered By

Muhammad Khurram
I have strong General Management skills to apply in your projects. Over last 3 years, I have acquired great knowledge of Accounting, Auditing, Microsoft Excel, Microsoft PowerPoint, Finance, Microsoft Project, Taxation, Strategic Management, Human Resource, Financial Planning, Business Planning, Microsoft Word, International Business, Entrepreneurship, General Management, Business Mathematics, Advertising, Marketing, Supply Chain, and E-commerce. I can guarantee professional services with accuracy.
4.80+
249+ Reviews
407+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
5. For the circuit in Figure below, use Thevenin's theorem to find the output voltage vo 3i in 2 H 492 www 292 12 cos(1) V F= 118 HH
-
You decide to help I. M. with his analysis. A good fax machine will cost $500 and functions properly for five years. The phone company charges $300 for installing a new line and $60 a month for the...
-
The ABC marketing consulting firm found that a particular brand of tablet PCs has the following demand curve for a certain region: Q = 10,000 - 200P + 0.03Pop + 0.6I + 0.2A where Q is the quantity...
-
You have just been given a $454,000, which you decide to invest at an APR of 6.7 percent. If you were to withdraw $38,500 at the end of each year, starting at the end of this year, how many years...
-
Mop and Broom Manufacturing estimates that it takes 4.5 hours for each broom to be produced, from raw materials to final product. An evaluation of the process reveals that the amount of time spent...
-
An airplane with an airspeed of 120 km/h encounters a 90-km/h crosswind. Convince your classmates that the plane's groundspeed is 150 km/h.
-
Water is pumped from the tank shown in Fig. P5.110a. The head loss is known to be \(1.2 V^{2} / 2 g\), where \(V\) is the average velocity in the pipe. According to the pump manufacturer, the...
-
Provide a concise summary of the five basic techniques used to manipulate, grow, examine, and characterize microorganisms in a laboratory.
-
You and your friend each have a graduated cylinder identical to the cylinder 2 from our class. You read a volume of 371mL. Your friend reads a volume of 381mL. On the basis of this information, can...
-
Aspirin, C 6 H 4 (OCOCH 3 )CO 2 H, is produced by the reaction of salicylic acid, C 6 H 4 (OH)CO 2 H, and acetic anhydride, (CH 3 CO) 2 O . If you mix 100. g of each of the reactants, what is the...
-
In the thermite reaction, iron(III) oxide is reduced by aluminum to give molten iron. If you begin with 10.0 g of Fe 2 O 3 and 20.0 g of Al, (a) Which reactant is limiting? (b) What mass of Fe can be...
-
A study by researchers at the University of Maryland addressed the question of whether the mean body temperature of humans is 98.6F. The results of the study by P. Mackowiak et al. appeared in the...
-
Your company began operations on the first day of the current month. Journal entries for the first month follow. Account Cash Debit 150,000 Credit Common stock 150,000 Land 95,000 Equipment 55,000...
-
What are the 3 basic components of every nucleotide? a. Write the complementary base sequence for the above sequence of DNA. b. Write the m RNA sequence for the original sequence of DNA.
-
How can leaders develop and maintain expert power in an ever-changing world where knowledge and expertise are constantly evolving. How do the Five Bases of Power apply to different contexts, such as...
-
Black Paua Inc. announced on May 1 that it will pay a dividend of $10 per share on June 15 to all holders on record as of May 31st. The firm's stock price closed today at $50 a share. Assume all...
-
Kelly has been feeling very sad for a month. Her sadness is so pronounced that she has not showered for days on end, sometimes has forgotten to eat, and has not been interested in doing anything....
-
What is a fiscal year? Why might companies choose to use a fiscal year that is not a calendar year?
-
2. In the circuit given in Figure 2, i,(t) = 5.67cos(5t)A and v (t) = 70.71 cos(5t 60) V a) Find the equivalent load impedance. State whether the load is inductive or capacitive. b) Calculate the...
-
Each of the following syntheses will not produce the desired product. In each case, identify the flaw in the synthesis. (a) (b) (c) (d) NO2 1) HNO3, H2SO, 2) EECI, AICI3 Et Br 1) Br2, FeBrz 2) AICI,
-
When para-bromotoluene is treated with sodium amide, two products are obtained. Draw both products, and propose a plausible mechanism for their formation.
-
Picric acid is a military explosive formed via the nitration of phenol under conditions that install three nitro groups. Draw the structure and provide an IUPAC name for picric acid.
-
Assume that four years and one month from today you plan to make the first of several annual withdrawals from an account. Your first withdrawal will equal $1000. You plan for these withdrawals to...
-
If I borrowed 15,000 in student loans at an annual interest of 7%. and then repay $1800 per year, then how long will it take me to repay the loan?
-
An investment offers the following cash flows: $554 today, $182 one year from now, $482 in 2 years, and $793 in 3 years. If the relevant interest rate is 7% per year (an APR, with interest compounded...

Study smarter with the SolutionInn App


