Isomers are molecules with the same elemental composition but a different atomic arrangement. Three isomers with the
Question:
Isomers are molecules with the same elemental composition but a different atomic arrangement. Three isomers with the formula C4H8 are shown in the models below. The enthalpy of combustion (∆cH°) of each isomer, determined using a calorimeter, is as follows: 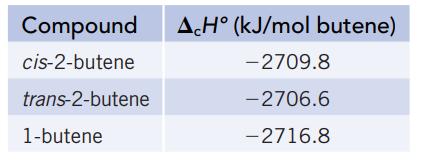
(a) Draw an energy level diagram relating the energy content of the three isomers to the energy content of the combustion products, CO2(g) and H2O(ℓ).
(b) Use the ∆cH° data in part (a), along with the enthalpies of formation of CO2(g) and H2O(ℓ) from Appendix L, to calculate the enthalpy of formation for each of the isomers.
(c) Draw an energy level diagram that relates the enthalpies of formation of the three isomers to the energy of the elements in their standard states.
(d) What is the enthalpy change for the conversion of cis-2-butene to trans-2-butene
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





