Metals generally react with hydrogen halides to give the metal halide and hydrogen. Determine whether this is
Question:
Metals generally react with hydrogen halides to give the metal halide and hydrogen. Determine whether this is true for silver by calculating ΔrG° for the reaction with each of the hydrogen halides.![]()
The required free energies of formation are: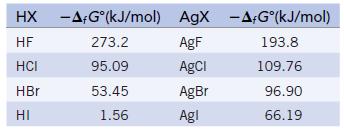
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





