Potassium hydrogen phthalate, KHC 8 H 4 O 4 , is used to standardize solutions of bases.
Question:
Potassium hydrogen phthalate, KHC8H4O4, is used to standardize solutions of bases. The acidic anion reacts with strong bases according to the following net ionic equation: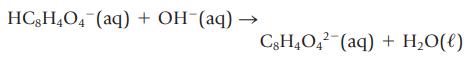
If a 0.902-g sample of potassium hydrogen phthalate is dissolved in water and titrated to the equivalence point with 26.45 mL of NaOH(aq), what is the molar concentration of the NaOH?
Transcribed Image Text:
HC8H4O4 (aq) + OH (aq) - C8H4042 (aq) + H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To find the molar concentration of NaOH sodium hydroxide you can use the concept of stoichiometry an...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A 0.288-g sample of an unknown monoprotic organic acid is dissolved in water and titrated with a 0.115 M sodium hydroxide solution. After the addition of 17.54 mL of base, a pH of 4.92 is recorded....
-
A 0.239-g sample of unknown organic base is dissolved in water and titrated with a 0.135 M hydrochloric acid solution. After the addition of 18.35 mL of acid, a pH of 10.73 is recorded. The...
-
A 25.00-mL sample contains 0.562 g of NaHCO3. This sample is used to standardize an NaOH solution. At the equivalence point, 42.36 mL of NaOH has been added. a. What was the concentration of the...
-
What is the timestep value ? And how do I go about altering from downstream to upstream? The following code solves the advection equation 1 2 3 4 5 6 7 8 9- 10 - 11 12 - 13 - 14 - 15 - 16 - 17 18 19...
-
A tire manufacturer has been concerned about the number of defective tires found recently. In order to evaluate the true magnitude of the problem, a production manager selected 10 random samples of...
-
Why does the color of sunsets vary from day to day?
-
A certain flow field is described by the stream function \[ \psi=A \theta+B r \sin \theta \] where \(A\) and \(B\) are positive constants. Determine the corresponding velocity potential and locate...
-
A monopolists inverse demand function is P = 150 - 3Q. The company produces output at two facilities; the marginal cost of producing at facility 1 is MC1 (Q1) = 6Q1, and the marginal cost of...
-
In her Ted talk, Kristi Rogers talks about the future of advertising and why it's crucial for ads to be relevant. She points out that even though we have lots of data and technology for digital ads,...
-
You have 0.954 g of an unknown acid, H 2 A, which reacts with NaOH according to the balanced equation If 36.04 mL of 0.509 M NaOH is required to titrate the acid to the second equivalence point, what...
-
If 38.55 mL of HCl is required to titrate 2.150 g of Na 2 CO 3 according to the following equation, what is the concentration (mol/L) of the HCl solution? NaCO3(aq) + 2 HCl(aq) - 2 NaCl(aq) + CO(g) +...
-
Find an organization that uses Agile techniques for requirements determination. What techniques do they use? How did they discover them? What did they use before? What is their evaluation of the...
-
Best Buy offers quantity discounts on Wireless Noise Cancelling headphones. The price for the first 30 Headphones is $40.00 per Headphone. After the first 30, all additional Headphones purchased, are...
-
Merger Analysis FIN490 | Understanding Merger Transactions Links to an external site. and FIN490 | Valuing Synergies in Transactions Links to an external site.. In your journal, Explain what the...
-
PolyProduction Inc. has two classes of common stock. Class A has 5 million shares with 10 votes per share. Class B has 5 million shares with 1 vote per share. If the dividends per share are equal for...
-
Reba the robot and Amby the automatic mopper each travel along the surface of the Earth from Ksar Kaddour, Algeria (at 30 N, 0 E) to Kikorongo, Uganda (at 0 N, 30 E). Reba travels at a constant speed...
-
Consider the function f(x) = In(4 - x). Determine: a. The domain of f(x). b. The x-intercept and y-intercept of the curve. C. The symmetry of the curve.
-
What is the formula to compute interest on a note receivable?
-
l ask this second time correnct answer is 38,01 can we look pls Consider a non-conducting rod of length 8.8 m having a uniform charge density 4.5 nC/m. Find the electric potential at P, a...
-
If the H o f for the chemical compounds involved in a reaction are available at a given temperature, how can H o R be calculated at another temperature?
-
A system undergoes a change from one state to another along two different pathways, one of which is reversible and the other of which is irreversible. What can you say about the relative magnitudes...
-
An ideal gas in a piston and cylinder assembly with adiabatic walls undergoes an expansion against a constant external pressure. Are S, S surroundings , and S total positive, negative, or zero?...
-
How would you suggest to your leaders or your colleagues that they should review the attached article and what would you expect them glean from the article?
-
QUESTION 2 (3.00 points) Develop a resource schedule for the project network below. The project involves a which requires a team of programmers for its development. However, only 5 programmers are...
-
1- Provide three specific reasons why companies should use expatriate managers to run those locations. 2- What three steps should a company take before sending an employee on an international...

Study smarter with the SolutionInn App


