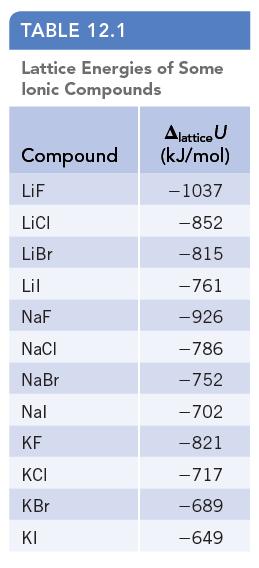
Prepare a graph of lattice enthalpy for lithium, sodium, and potassium halides (Table 12.1) vs. the sum
Question:
Prepare a graph of lattice enthalpy for lithium, sodium, and potassium halides (Table 12.1) vs. the sum of the ionic radii for the component ions (Figure 7.11). Evaluate the results and comment on the relationship between these quantities.
Data given in Table 12.1
 Data given in Figure 7.11
Data given in Figure 7.11

Transcribed Image Text:
TABLE 12.1 Lattice Energies of Some lonic Compounds Compound LiF LICI LiBr Lil NaF NaCl NaBr Nal KF KCI KBr KI Alattice U (kJ/mol) - 1037 -852 -815 -761 -926 -786 -752 -702 -821 -717 -689 -649
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Axial loads are applied with rigid bearing plates to the solid cylindrical rods shown in the figure. One load of P = 34 kips is applied to the assembly at A, two loads of Q = 30 kips are applied at...
-
The file Fortune500 contains data for profits and market capitalizations from a recent sample of firms in the Fortune 500 a. Prepare a scatter diagram to show the relationship between the variables...
-
The following data represent enrollment in a major at your university for the past six semesters (Note: semester 1 is the oldest data; semester 6 is the most recent data): Semester Enrollment 1.. 87...
-
Over the last five years, corporation A has been consistently profitable. Its earnings before taxes were as follows: Year 1 2 3 4 5 Earnings $1,300 $3,100 $4,000 $5,300 $4,500 If the corporate tax...
-
Debate the issue of Coca-Cola diversifying into non-drink products, such as PepsiCo had done quite successfully.
-
A bootstrap distribution of mean commute times (in minutes) based on a sample of 500 St. Louis workers stored in CommuteStLouis is shown in Figure 5.13. The pattern in this dotplot is reasonably...
-
Explain briefly how the scope of internal environmental audits has developed over the past 20 or so years.
-
Reena Corp. lost most of its inventory in a fire in December just before the year-end physical inventory was taken. The corporation's books disclosed the following: Merchandise with a selling price...
-
On January 1, 2024, Kingbird Company sold property to Ivanhoe Company which originally cost Kingbird $2750000. There was no established exchange price for this property. Ivanhoe gave Kingbird a...
-
Outline a procedure to calculate the percent of space occupied by the atoms in an fcc arrangement.
-
Spinels are described in Study Question 53. Consider two normal spinels, CoAl 2 O 4 and SnCo 2 O 4 . What metal ions are involved in each? What are their electron configurations? Are the metal ions...
-
The William Robertson Society is a charitable organization funded by government grants and private donations. It prepares its annual financial statements using the restricted fund method in...
-
This section of SOX established a quasi-governmental entity called the Public Company Accounting Oversight Board (PCAOB, but called "Peek-a-Boo") under the direction of the SEC to (1) oversee the...
-
Tim Donaghy, a referee for the NBA, entered a guilty plea to two federal felony charges in connection with his bets and tips on NBA games. The charges are conspiracy to engage in wire fraud and...
-
The governor's report cited three key reasons that such levels of cheating flourished in APS. The first was that the district set unrealistic test-score goals, or "targets." \({ }^{\text {" } 752}\)...
-
Under the leadership of CEO Don Blankenship, Massey went from a family-operated company to a corporation with 150 mines with revenues of \(\$ 2.6\) billion. That growth came in response to Mr....
-
Piper High School is in Piper, Kansas, a town located about 20 miles west of Kansas City, Missouri. Christine Pelton was a high school science teacher there. Ms. Pelton, age 26, had a degree in...
-
What are the characteristics of accrual accounting that allow managers to manage earnings? Why do these characteristics allow earnings to be managed?
-
For all of the following words, if you move the first letter to the end of the word, and then spell the result backwards, you will get the original word: banana dresser grammar potato revive uneven...
-
The boom is supported by the winch cable that has a diameter of 0.25 in. and an allowable normal stress of Ï allow = 24 ksi. Determine the greatest weight of the crate that can be supported...
-
The boom is supported by the winch cable that has an allowable normal stress of Ï allow = 24 ksi. If it supports the 5000 lb crate when f = 20°, determine the smallest diameter of the cable...
-
The assembly consists of three disks A, B, and C that are used to support the load of 140 kN. Determine the smallest diameter d 1 of the top disk, the largest diameter d 2 of the opening, and the...
-
8. Given the following code sequence: for (i=0; i <10; i++) { if (A[i] != 5) A[B[i]] += 1 else A[i] = B[i+1]} If the base address of arrays A and B are in $s1 and $s2 respectively and i, 5 and 1 are...
-
Using actual sales data, create a flexible budget. Using that flexible budget determine the flexible budget food expense, the labor expenses including cooks, cashiers, and servers (remembering the...
-
1. Find the statement that causes the compilation error. 2. Determine the output if that statement is removed. class A { public void test() { System.out.print("A"); } } class B extends A { public...

Study smarter with the SolutionInn App


