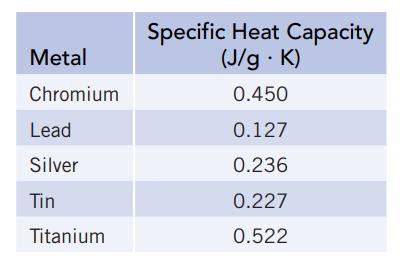
Prepare a graph of specific heat capacities for metals versus their atomic weights. Combine the data in
Question:
Prepare a graph of specific heat capacities for metals versus their atomic weights. Combine the data in Figure 5.4 and the values in the following table. What is the relationship between specific heat capacity and atomic weight? Use this relationship to predict the specific heat capacity of platinum. The specific heat capacity for platinum is given in the literature as 0.133 J/g ∙ K. How good is the agreement between the predicted and actual values?
Data given in Figure 5.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





