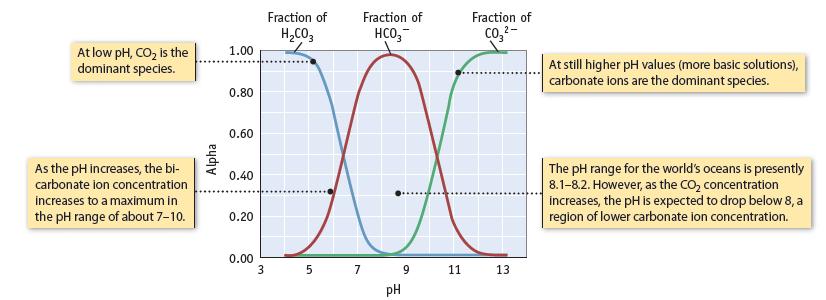
Refer to the figure below and Figure 20.25, which show the fraction of species in solution [alpha
Question:
Refer to the figure below and Figure 20.25, which show the fraction of species in solution [alpha (α)] as a function of pH. The following questions are in regard to the equilibria involved in an aqueous solution of H2CO3 (dissolved CO2) in fresh water at 25°C.
(a) At what pH does [H2CO3(aq)] = [HCO3−]?
(b) At what pH does [HCO3−] = [CO32−]?
(c) What is the predominant species in solution when the solution has a pH of 8?
(d) What species are in solution when the solution has a pH of 7?
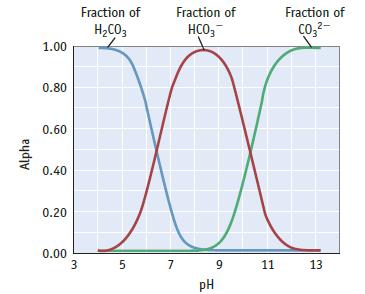
Data given in Figure 20.25
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





