The acidity of the oxoacids was described in Section 16.9, and a larger number of acids are
Question:
The acidity of the oxoacids was described in Section 16.9, and a larger number of acids are listed in the table below.
(a) What general trends do you see in these data?
(b) What has a greater effect on acidity, the number of O atoms bonded directly to the central atom E or the number of OH groups?
(c) Look at the acids based on Cl, N, and S. Is there a correlation of acidity with the formal charge on the central atom, E?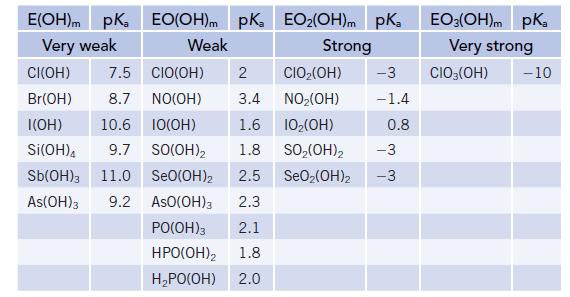
(d) The acid H3PO3 has a pKa of 1.8, and this led to some insight into its structure. If the structure of the acid were P(OH)3, what would be its predicted pKa value? Given that this is a diprotic acid, which H atoms are lost as H+ ions?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





