The molecule shown here, 2-furylmethanethiol, is responsible for the aroma of coffee: (a) What are the formal
Question:
The molecule shown here, 2-furylmethanethiol, is responsible for the aroma of coffee: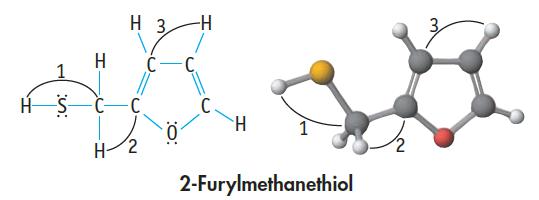
(a) What are the formal charges on the S and O atoms?
(b) Give approximate values of angles 1, 2, and 3.
(c) Which are the shorter carbon–carbon bonds in the molecule?
(d) Which bond in this molecule is the most polar?
(e) Is the molecule polar or nonpolar?
(f) The four C atoms of the ring are all in a plane. Is the O atom in that same plane (making the five-member ring planar), or is the O atom bent above or below the plane?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





