The reaction of methane and water is one way to prepare hydrogen for use as a fuel:
Question:
The reaction of methane and water is one way to prepare hydrogen for use as a fuel:
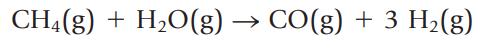
If you begin with 995 g of CH4 and 2510 g of water,
(a) Which reactant is the limiting reactant?
(b) What is the maximum mass of H2 that can be prepared?
(c) What mass of the excess reactant remains when the reaction is completed?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





