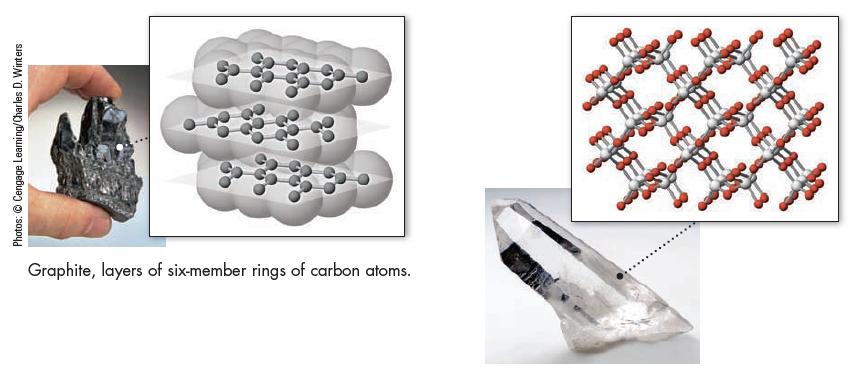
The structure of graphite is given in Figure 12.19. (a) What type of intermolecular forces exist between
Question:
The structure of graphite is given in Figure 12.19.
(a) What type of intermolecular forces exist between the layers of six-member carbon rings?
(b) Account for the lubricating ability of graphite. That is, why does graphite feel slippery? Why does pencil lead (which is really graphite in clay) leave black marks on paper?
Data given in Figure 12.19
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





