The structure of vitamin C, whose chemical name is ascorbic acid, is drawn below (without lone pairs
Question:
The structure of vitamin C, whose chemical name is ascorbic acid, is drawn below (without lone pairs of electrons).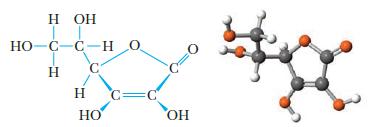
(a) What is the approximate value for the O—C—O bond angle?
(b) There are four OH groups in this structure. Estimate the C—O—H bond angles for these groups. Will they be the same value (more or less), or should there be significant differences in these bond angles?
(c) Is the molecule chiral? How many chiral carbon atoms can be identified in this structure?
(d) Identify the shortest bond in this molecule.
(e) What are the functional groups of the molecule?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





