The transition metals form a class of compounds called metal carbonyls, an example of which is the
Question:
The transition metals form a class of compounds called metal carbonyls, an example of which is the tetrahedral complex Ni(CO)4. Given the following thermodynamic data (at 298 K):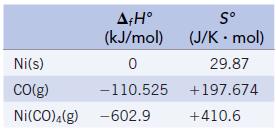
(a) Calculate the equilibrium constant for the formation of Ni(CO)4(g) from nickel metal and CO gas.
(b) Is the reaction of Ni(s) and CO(g) product- or reactant-favored at equilibrium?
(c) Is the reaction more or less product-favored at higher temperatures? How could this reaction be used in the purification of nickel metal?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





