Use data in Appendix L to calculate the enthalpy and free energy change for the reaction 2
Question:
Use data in Appendix L to calculate the enthalpy and free energy change for the reaction 2 NO2(g) → N2O4(g)
Is this reaction exothermic or endothermic? Is the reaction product- or reactant-favored at equilibrium?
Data given in Appendix L
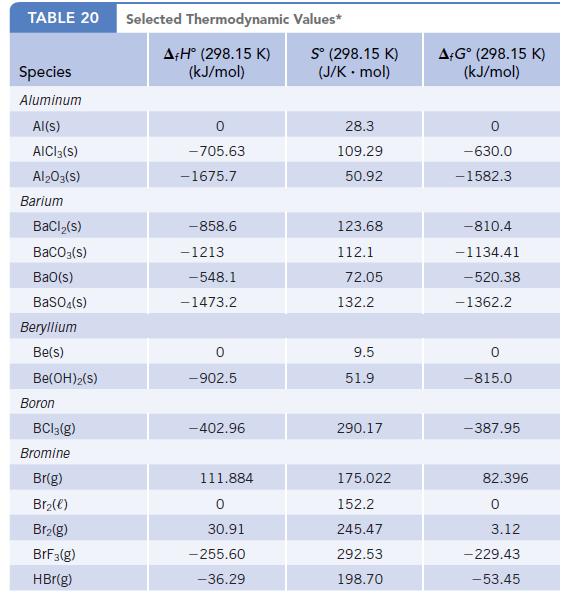
Transcribed Image Text:
TABLE 20 Species Aluminum Al(s) AICI 3(S) Al2O3(S) Barium BaCl₂(s) BaCO3(s) BaO(s) BaSO4(s) Beryllium Be(s) Be(OH)2(S) Boron BC13(g) Bromine Br(g) Br₂(e) Br₂(g) BrF3(g) HBr(g) Selected Thermodynamic A+Hº (298.15 K) (kJ/mol) 0 -705.63 -1675.7 -858.6 -1213 -548.1 -1473.2 -902.5 -402.96 111.884 0 30.91 -255.60 -36.29 Values* Sº (298.15 K) (J/K . mol) 28.3 109.29 50.92 123.68 112.1 72.05 132.2 9.5 51.9 290.17 175.022 152.2 245.47 292.53 198.70 A+Gᵒ (298.15 K) (kJ/mol) -630.0 -1582.3 -810.4 -1134.41 -520.38 -1362.2 0 -815.0 -387.95 82.396 0 3.12 - 229.43 -53.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
2 NO NO4 AH for NO22331662 KJmol AH for NO 908 KJmol AS for NO2 24004JmolK 28 AS ...View the full answer

Answered By

Dharmpal Kumar
i have done graduation in electrical engineering from cochin university of science and technology.i have 2years of teaching experience and have cracked various competitive exam like GATE and IIT JEE .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Use data in Appendix L to calculate the enthalpy and free energy change for the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g). Is this reaction exothermic or endothermic? Is the reaction product- or...
-
The free energy change for a reaction G is an extensive property. What is an extensive property? Surprisingly, one can calculate G from the cell potential for the reaction. This is surprising because...
-
The decomposition of crystalline N2O5 N2O5(s) 2NO2(g) + 1/2 O2(g) is an example of a reaction that is thermodynamically favored even though it absorbs heat. At 25C we have the following values for...
-
Boneyard Biscuits Dutch auction for an IPO was a great success. The firm offered 100 million shares. Bids appear below. a. What is the clearing price? b. What options do Boneyard and its underwriters...
-
Distinguish between (1) imposing risk on others by driving carelessly without an accident actually occurring, and (2) inspiring fear in others by attempting to commit a crime and failing.
-
The capacitor in the circuit of Fig. 16.39 is initially uncharged. Find v 0 (t) for t > 0. 4i 9.68(t) V (+ 1F Vo
-
When light falls on a balanced detector (i.e., a detector pair, with one detector output subtracted from the other's output), the output current is proportional to the difference of the intensities...
-
See Table 2.5 showing financial statement data and stock price data for Mydeco Corp. Was Mydeco able to improve its ROIC in 2013 relative to what it was in 2009? Table continue TABLE 2.5 2009-2013...
-
The following events occurred for Johnson Company: a. Received investment of cash by organizers and distributed to them 1,010 shares of $1 par value common stock with a market price of $40 per share....
-
A major use of hydrazine, N 2 H 4 , is in steam boilers in power plants. (a) The reaction of hydrazine with O 2 dissolved in water gives N 2 and water. Write a balanced equation for this reaction....
-
The halogen oxides and oxoanions are good oxidizing agents. For example, the reduction of bromate ion has an E value of 1.44 V in acid solution: Is it possible to oxidize aqueous 1.0 M Mn 2+ to...
-
A galvanometer having a resistance of 25.0 has a 1.00 shunt resistance installed to convert it to an ammeter. It is then used to measure the current in a circuit consisting of a 15.0 resistor...
-
Joey offers to sell Melissa the latest version of a popular gaming system for only $25 cash, although in the store it generally costs over $200. The deal seems too good to be true, and Melissa...
-
Carrie is addicted to heroin and will do anything to feed her addiction. She decides to take her husbands Rolex watch and sell it at the local pawnshop for cash. The Rolex watch was purchased with...
-
False pretenses is shorthand for the crime of obtaining property by deception. It is distinct from larceny because of the deception and/or false representation involved. Forgery is the false making...
-
When a piece of cork floats in water, three-quarters of its volume is above the water surface. How does the mass density of cork compare with the mass density of water?
-
A dam of horizontal length \(\ell\) holds water of mass density \(ho\) to a height \(h\). What is the magnitude of the force exerted by the water on the dam?
-
Problem described some adjustments made by Susan Hatfield, CPA. Prepare the necessary adjustment as it would be made by the client in transactions (2) and (3), and by the secretary in transaction...
-
What are the risks and liability factors in an audit? What are the implications to the auditor? What are the implications to the organization? How can the auditor mitigate these risks and liability...
-
A vector A(vector) has a magnitude of 15 (in some unspecified units) and makes an angle of 25 with the x axis, and a vector B(vector) has a magnitude of 25 and make an angle of 70 with the x axis....
-
Consider the vector C(Vector) in Figure P1.55. Construct a drawing that shows the vector C(Vector) along with the vector 4C(Vector), - C(Vector), and - 3C(Vector). Figure P1.55 y
-
Consider a map oriented so that the x axis runs eastwest (with east being the positive direction) and y runs northsouth (with north positive). A person drives 25 km to the north, turns and drives 75...
-
Aronian Import received an invoice dated January 5 for a shipment of goods received January 11. The invoice was for $ 65525 less 8% with terms 3/20 R.O.G. (a)How much should Aronian Import pay on...
-
In the figure given below, O is the centre of the circle and AB is a diameter. A 72% B If AC BD and LAOC = 72. Find: (i) ZABC (ii) ZBAD (iii) ZABD D Lear
-
Golden Corp. is a young start-up company and therefore is not paying any dividends on the stock over the next 8 years. At the end of year 8, the company will pay a $5 dividend. The following year,...

Study smarter with the SolutionInn App


