Write the molecular formula and calculate the molar mass for each of the molecules shown here. Which
Question:
Write the molecular formula and calculate the molar mass for each of the molecules shown here. Which has the largest mass percent of carbon? Of oxygen?
(a) Ethylene glycol (used in antifreeze)
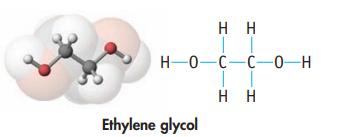
(b) Dihydroxyacetone (used in artificial tanning lotions)
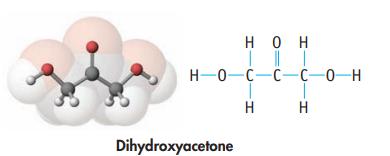
(c) Ascorbic acid, commonly known as vitamin C 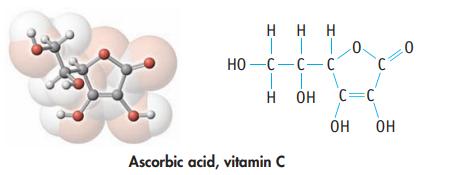
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





