A chemical engineer is working to optimize the production of acrylonitrile to be used in the manufacture
Question:
A chemical engineer is working to optimize the production of acrylonitrile to be used in the manufacture of carbon fibers. The reaction being used is the combination of propene gas, ammonia, and oxygen. The reaction is normally carried out at moderately high temperatures so all species are in the gas phase.
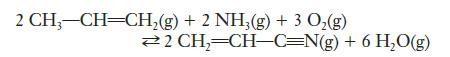
(a) Write the equilibrium constant expression for this reaction.
(b) The boiling point of acrylonitrile is 77°C, and that of propene is −48°C. What would the equilibrium expression be if this reaction were carried out at room temperature?
(c) What characteristic of this reaction might cause the engineer to desire carrying out this reaction at room temperature?
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





