A chemical engineering student is studying the effect of pH on the corrosion of iron. The following
Question:
A chemical engineering student is studying the effect of pH on the corrosion of iron. The following data are collected:
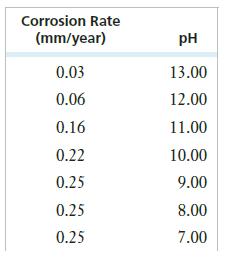
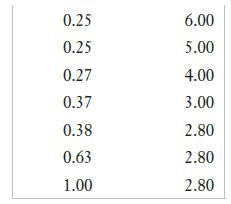
Plot corrosion rate vs. pH. ( Label the x axis with decreasing pH.)
(a) How would you describe the dependence of corrosion on pH?
(b) When the pH goes below 3, bubbles appear in the solution. What change takes place in the reduction half-reaction at low pH?
(c) How do you explain the shape of the curve at the lowest pH?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





