Question: An experiment is designed to test a component in a natural gas processing plant. To mimic the raw natural gas, a mixture is prepared with
An experiment is designed to test a component in a natural gas processing plant. To mimic the raw natural gas, a mixture is prepared with the mole fractions given in the following table.
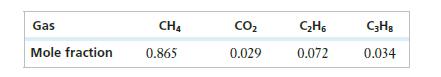
If the desired total pressure is 750 torr, what should the partial pressures be? If the gas is to be in a 15.0-L vessel held at 30°C, how many moles of each substance are needed?
Strategy We know the mole fractions and the desired total pressure. So we can calculate partial pressures using the relationship defined in Equation 5.10. From the total pressure and the volume, we can calculate the total number of moles and thereby the number of moles of each gas.
Equation 5.10.
![]()
Gas Mole fraction CH4 0.865 CO 0.029 CH6 0.072 C3H8 0.034
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Check Your Understanding A mixture of C 2 H 6 g and C 3 H 8 g is ... View full answer

Get step-by-step solutions from verified subject matter experts


