Each of the following monomers or pairs of monomers can undergo condensation polymerization reactions. Draw the structures
Question:
Each of the following monomers or pairs of monomers can undergo condensation polymerization reactions. Draw the structures showing the repeat units and linkages in each of the resulting polymers.
(a) glycine:
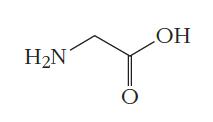
(b) 6-aminocaproic acid:
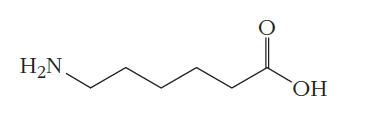
(c) p-phenylenediamine and terephthalic acid:
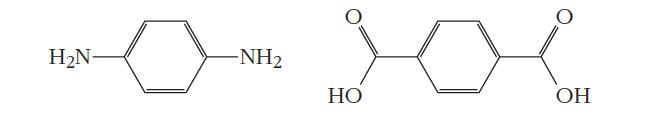
Strategy Condensation polymerization involves the elimination of a small molecule— usually water—as each monomer is added to the growing chain. We can look for functional groups on the ends of the monomers that can undergo such a reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





