Hydrazine, N 2 H 4 , has been proposed as the fuel in a fuel cell in
Question:
Hydrazine, N2H4, has been proposed as the fuel in a fuel cell in which oxygen is the oxidizing agent. The reactions are
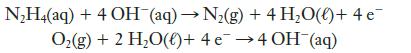
(a) Which reaction occurs at the anode and which at the cathode?
(b) What is the net cell reaction?
(c) If the cell is to produce 0.50 A of current for 50.0 h, what mass in grams of hydrazine must be present?
(d) What mass in grams of O2 must be available to react with the mass of N2H4 determined in part (c)?
Transcribed Image Text:
N₂H4(aq) + 4 OH¯(aq) → N₂(g) + 4H₂O(l) + 4e¯ O₂(g) + 2 H₂O()+ 4 e 4 OH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Which reaction occurs at the anode and which at the cathode The anode is the negative electrode an...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The large magnetic fields used in MRI can produce forces on electric currents within the human body. This effect has been proposed as a possible method for imaging biocurrents flowing in the body,...
-
A study is to be made using liquid ammonia as the fuel in a gas-turbine engine. Consider the compression and combustion processes of this engine. a. Air enters the compressor at 100 kPa, 25C, and is...
-
A study is to be made using liquid ammonia as the fuel in a gas-turbine engine. Consider the compression and combustion processes of this engine. a) Air enters the compressor at 100 kPa, 25C,...
-
If you could choose, which type of school would you want your imaginary child(ren) to attend?
-
Compare and contrast the methodologies used by Interbrand (www.interbrand.com) and BrandZ (www.brandz.com) to determine brand value. Explain why there is a discrepancy in the rankings from these two...
-
JoKatherine Page and her 14-year-old son Jason were robbed at their banks ATM at 9:30 P.M. one evening by a group of four thugs. The thieves took $300, struck Mrs. Page in the face with a gun, and...
-
Follow the steps below to prove the LLN without using CLT. (a) Let \(X\) be a random variable with mean \(\mu\) and variance \(\sigma^{2}\). Then for any real number \(\alpha>0,...
-
The following are selected 2014 transactions of Yosuke Corporation. Jan. 1 Purchased a small company and recorded goodwill of $150,000. Its useful life is indefinite. May 1 Purchased for $84,000 a...
-
Westeros, Inc. receives scabbards (sheath for a sword) from two different suppliers. 70 percent of its scabbards come from Knights Watch, LLC, while 30 percent come from Crow, Inc. Records indicate...
-
Suppose that, prior to other firms entering the market, the maker of a new smartphone (Way Cool, Inc.) earns $100 million per year. By reducing its price by 50 percent, Way Cool could discourage...
-
A current is passed through a solution of copper(II) sulfate long enough to deposit 14.5 g of copper. What volume of oxygen is also produced if the gas is measured at 24C and 0.958 atm of pressure?
-
The following information is taken from the records of O'Donnell Corp. at June 30, 2016, its fiscal year-end: Advertizing expense Commissions expense Delivery expense Insurance expense Opening...
-
What is the difference between reciprocation and mutualism? Explain briefly
-
You sell inventory items to customers in the same way you sell a service. What is a discount? How do you activate discounts?
-
How is the break-even point calculated for a percentage lease? Unset starred question Divide the annual base rent by the percentage the landlord established. Divide the annual expenses by the monthly...
-
Find points of inflection and discuss concavity for f(x)=x(x-4) 3 +k. Each student should post their new equation for f(x) as the first line of their post. k=19,
-
Explain what is meant by sensitivity analysis in contribution analysis and state the advantages in using such a technique.
-
A cantilever beam with a 1-in-diameter round cross section is loaded at the tip with a transverse force of 1000 lbf, as shown in the figure. The cross section at the wall is also shown, with labeled...
-
Why can wastewater treatment requirements in Hawaii be less stringent than those in most locations on the U.S. mainland?
-
The gear motor can develop 2 hp when it turns at 150 rev/min. If the allowable shear stress for the shaft is Ï allow = 8 ksi, determine the smallest diameter of the shaft to the nearest 1/8 in....
-
The gear motor can develop 1/4 hp when it turns at 600 rev/min. If the shaft has a diameter of 1/2 in., determine the maximum shear stress in the shaft.
-
The gear motor can develop 1/10 hp when it turns at 80 rev/min. If the allowable shear stress for the shaft is Ï allow = 4 ksi, determine the smallest diameter of the shaft to the nearest 1/8...
-
The following is a schematic micrograph that represents the microstructure of some hypothetical metal. Determine the following: (a) Mean intercept length (b) ASTM grain size number, G (a) = i (b) G =...
-
3. How many electrons are permitted in a(n) 1s orbital? 2s orbital? 5s orbital? 2p orbital? 3p orbital? 4p orbital? 3d orbital? 4d orbital? 5d orbital? 4f orbital? 5f orbital?
-
A pilot experiment using a filter of 0.1 m area at a constant pressure of 68.5-104 Pa produced the results on the table below. The filtrate viscosity was 0.0015 Pas, slurry concentration was 3% w/w,...

Study smarter with the SolutionInn App


