Ironaluminum alloys are useful in some applications because they become magnetized in a magnetic field but are
Question:
Iron–aluminum alloys are useful in some applications because they become magnetized in a magnetic field but are easily demagnetized when the field is removed. The composition of an iron–aluminum alloy can be determined chemically by reacting it with hydrochloric acid:
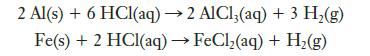
When a 7.264-g sample of a particular iron–aluminum alloy was dissolved in excess hydrochloric acid, 0.3284 g of H2(g) was produced. What was the mass percentage of aluminum in the alloy?
Transcribed Image Text:
2 Al(s) + 6 HCl(aq) → 2 AlCl3(aq) + 3 H₂(g) Fe(s) + 2 HCl(aq) → FeCl₂(aq) + H₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To find the mass percentage of aluminum in the ironaluminum alloy we need to use the information pro...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
22+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Established in 1980, Pizza Station is located in the trendy downtown area of Salina, Pennsylvania. Situated within walking distance of Salina State College, the restaurant initially offered in-house...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Jogger 1 is travelling east at 6 . 5 m / s and has a mass of 8 2 kg . Jogger 2 is travelling north at 5 . 8 m / s and has a mass of 5 4 . 5 kg . One of the joggers has their head down and doesnt see...
-
Classify each of the following activities within a tax-preparation business as value-added (VA) or nonvalue-added (NVA). 1. Advertising. 2. Completing tax returns. 3. Cleaning the office. 4. Billing...
-
Tribeck Company is a family-owned business in which you own 20% of the common stock and your brothers and sisters own the remaining shares. The employment contract of Tribeck's new president, Jake...
-
The Anchor Glass Container Corporation and its parent company, Consumers Packaging, Inc. (CPI), entered into a series of agreements with Encore Glass, Inc., to supply glass containers of a specific...
-
Rockland Corporation earned net income of $300,000 in 2010 and had 100,000 shares of common stock outstanding throughout the year. Also outstanding all year was $800,000 of 10% bonds, which are...
-
35. Expandiendo e sin en serie de Fourier en wt, mostrar que la ecuacin trascendedente de Kepler tiene la solucin formal: = wt+Jn(ne) sin (wt), n=1 n 2 donde J, es la funcin de Bessel de orden n....
-
A mixture of methane (CH 4 ) and propane (C 3 H 8 ) has a total mass of 29.84g. When the mixture is burned completely in excess oxygen, the CO 2 and H 2 O products have a combined mass of 142.97g....
-
Calcium sulfate is the essential component of plaster and sheet rock. Waste calcium sulfate can be converted into quicklime, CaO, by reaction with carbon at hightemperatures. The following two...
-
The coefficient of static friction between Teflon and scrambled eggs is about 0.04. What is the smallest angle from the horizontal that will cause the eggs to slide across the bottom of a...
-
Natural gas from the well contains significant amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), which must be removed to produce pipeline quality gas. A typical gas cleaning plant uses a...
-
2. (4 points) Order the following stages of the demographic transition by putting a number next to each of the unordered stages below (1 is the first stage, 2 is the second stage, etc.). If a stage...
-
Given vector u = (4,-2) and v u and v of the form au + bv that would result in the vector (-4,-10). = (2, 2), find a linear combination of the vectors
-
A multimode step-index fibre has a core index of 1.484 and a cladding index of 1.456. When the fibre is overfilled with light emitted from a light-emitting diode (LED), estimate the pulse broadening...
-
A disabled supertanker ship is pulled by three tugboats, A, B and C, each pulling in a different direction, as shown in the figure. Tugboat A pulls with a force A of magnitude 1.2 x 106 N. Similarly,...
-
Brain and Nui Soon live in Macon, Georgia. Two years ago, they visited Thailand. Nui, a professional chef, was impressed with the cooking methods and the spices used in the Thia food. Macon does not...
-
Which one of the following anhydrous chloride is not obtained on direct heating of its hydrated chloride? (A) BaCl2 (B) CaClz (C) MgCl2 (D) SrCl2
-
The vapor pressure of an unknown solid is approximately given by ln(P/Torr) = 22.413 2211 (K/T), and the vapor pressure of the liquid phase of the same substance is approximately given by ln(P/Torr)...
-
For water, H vaporization is 40.656 kJmol 1 , and the normal boiling point is 373.12 K. Calculate the boiling point for water on the top of Mt. Everest of (elevation 8848 m), where the normal...
-
Calculate the difference in pressure across the liquidair interface for a (a) Mercury and (b) Methanol droplet of radius 125 nm.
-
1. Find the mean, median and mode(s) of each of the following sets of data. a. 4, 6, 9, 3, 4, 3,2 b. 26, 32, 10, 16, 11, 21, 13, 16, 21 C. 8, 10, 10, 10, 11, 11, 11, 15, 19, 23 d. The following...
-
How many red photons of wavelength 806 nm would it take to raise the temperature of 1g of water 1 degree Celsius? How many blue photons of 428 nm would it take to do the same?
-
Larry Bar opened a frame shop and completed these transactions: 1. Larry started the shop by investing $41,100 cash and equipment valued at $19,100. Purchased $180 of office supplies on credit. 2. 3....

Study smarter with the SolutionInn App


