The first four energy levels for the hydrogen atom are shown in the diagram below. The lowest
Question:
The first four energy levels for the hydrogen atom are shown in the diagram below. The lowest energy state is labeled E1, and the higher states are labeled E2, E3, and E4, respectively. When a hydrogen atom undergoes a transition from E3 to E1, it emits a photon with λ = 102.6 nm. Similarly, if the atom undergoes a transition from E3 to E2, it emits a photon with λ = 656.3 nm. Find the wavelength of light emitted by an atom making a transition from E2 to E1.
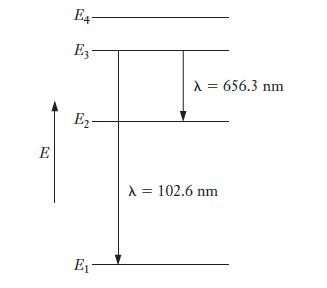
Strategy The energy-level diagram shows us the relationship between the various energy levels involved. The vertical direction in the diagram is energy. So, if we represent each transition by an arrow, as shown, then the length of that arrow will be proportional to the energy lost by the atom in that transition. From the diagram, we can see that the lengths of the arrows for the E3 to E2 transition and for the E2 to E1 transition must add up to the length of the arrow for the E3 to E1 transition. (Another way of thinking about this is that an atom going from the E3 state to the E1 state must lose the same amount of energy whether or not it stops at E2 along the way.) Because we know the wavelengths for two of the transitions, we can find the corresponding photon energies. That will let us find the energy for the third transition, and then finally we can convert that back to wavelength.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





