Question: Using data from Table 6.5 (page 204), predict the ions that magnesium and aluminum are most likely to form. Table 6.5 Strategy Scan the successive
Using data from Table 6.5 (page 204), predict the ions that magnesium and aluminum are most likely to form.
Table 6.5
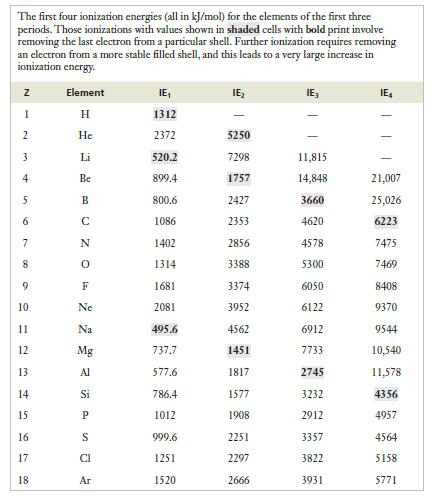
Strategy Scan the successive ionization energies for each element to find a point were removing one additional electron causes a dramatic increase in the value. It is not likely that sufficient energy would be available to compensate for such a large increase in ionization energy, so the ion formed will be dictated by the number of electrons lost before that jump in ionization energy.
The first four ionization energies (all in kJ/mol) for the elements of the first three periods. Those ionizations with values shown in shaded cells with bold print involve removing the last electron from a particular shell. Further ionization requires removing an electron from a more stable filled shell, and this leads to a very large increase in ionization energy. N 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Element H He Li Be B N 0 F Ne Na Mg Al Si P CI Ar IE 1312 2372 520.2 899.4 800.6 1086 1402 1314 1681 2081 495.6 737.7 577.6 786.4 1012 999.6 1251 1520 IE 5250 7298 1757 2427 2353 2856 3388 3374 3952 4562 1451 1817 1577 1908 2251 2297 2666 IE 11,815 14,848 3660 4620 4578 5300 6050 6122 6912 7733 2745 3232 2912 3357 3822 3931 IE 1 21,007 25,026 6223 7475 7469 8408 9370 9544 10,540 11,578 4356 4957 456 5158 5771
Step by Step Solution
3.25 Rating (151 Votes )
There are 3 Steps involved in it
For magnesium the first significant jump occurs between the second ionization energy 1451 kJmol and ... View full answer

Get step-by-step solutions from verified subject matter experts


