Question: A 0.500-g sample of a nonvolatile, yellow crystalline solid dissolves in 15.0 g benzene, producing a solution that freezes at 5.03 C. Use the data
A 0.500-g sample of a nonvolatile, yellow crystalline solid dissolves in 15.0 g benzene, producing a solution that freezes at 5.03 °C. Use the data in Table 12.4 to find the molar mass of the yellow solid.
Table 12.4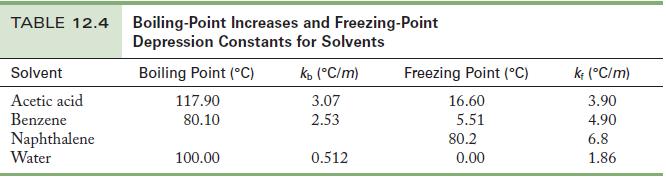
Step by Step Solution
★★★★★
3.34 Rating (151 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Heres how to find the molar mass of the yellow solid 1 The freezing point of pure benzene is 551 C f... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


