A 40.0-g sample of gold powder at 91.50 C is dissolved into 51.2 g mercury that is
Question:
A 40.0-g sample of gold powder at 91.50 °C is dissolved into 51.2 g mercury that is initially 22.00 °C. Using the specific heats given in Table 5.1, calculate the final temperature of the resulting solution, called an amalgam.
Table 5.1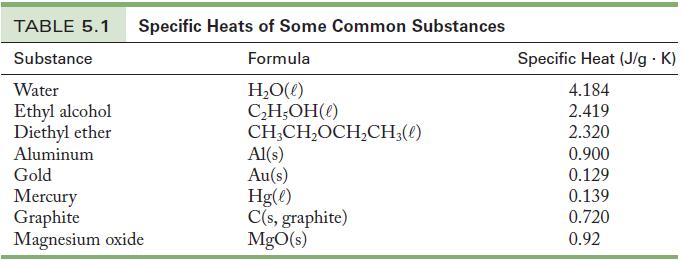
Transcribed Image Text:
TABLE 5.1 Specific Heats of Some Common Substances Substance Formula H₂O(l) C₂H,OH(1) CH₂CH₂OCH₂CH3(1) Water Ethyl alcohol Diethyl ether Aluminum Gold Mercury Graphite Magnesium oxide Al(s) Au(s) Hg(l) C(s, graphite) MgO(s) Specific Heat (J/g. K) 4.184 2.419 2.320 0.900 0.129 0.139 0.720 0.92
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To find the final temperature of the resulting amalgam after dissolving gold into mercury we can use the principle of conservation of energy The heat ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What is, according to Thomas Mun, the ordinary way for a Kingdom (which does not have mines) to increase its wealth? Explain.
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Create graphs similar to Fig. 14-5, but for lead and ethyl alcohol. Compare and contrast them with each other and with the graph for water. Are there any temperature ranges for which all three...
-
Which of the following is not necessary to do before you can run a Java program? a. Coding b. Compiling c. Debugging d. Saving
-
Air at 1 atm, 200F, and a relative humidity of 15% enters a direct-heat dryer. Determine the following from the psychrometric chart and/or relationships of Table 18.3. (a) Wet-bulb temperature. (b)...
-
Only one of these calculations is correct. Which one? Why are the others wrong? Give reasons for your answers. a. b. c. d. lim x ln x = 0(-) = 0 x->0+
-
Pereira Brick Pizza Ovens sells custom built stone and brick outdoor pizza ovens on both a credit and direct debit basis. They stock a full range of spare parts and accessories for preparing and...
-
Boyce Company's beginning inventory and purchases during the fiscal year ended September 30, 20-2, were as follows: Use the following information for the specific identification method. There are 900...
-
* Installing integrated software needs more memory than installing many .standalone applications True O False
-
The following six-column table for Solutions Co. includes the unadjusted trial balance as of December 31, 2013. Required 1. Complete the six-column table by entering adjustments that reflect the...
-
A 50.0-g sample of metal at 100.00 C is added to 60.0 g water that is initially 25.00 C. The final temperature of both the water and the metal is 31.51 C. (a) Use the specific heat of water to fi nd...
-
A 50.0-g sample of metal at 100.00 C is added to 40.0 g water that is initially 23.50 C. The final temperature of both the water and the metal is 28.46 C. (a) Use the specific heat of water to fi nd...
-
The first histogram below shows the distribution of the yearly incomes of 40 patrons at a college coffee shop. Suppose two new people walk into the coffee shop: one making $225,000 and the other...
-
Sketch the curves using the equations given in Problems 28-51. \(\frac{x^{2}}{25}+\frac{y^{2}}{36}=1\)
-
Graph the first-degree inequalities in two unknowns in Problems 13-48. \(x+3 y>420\)
-
Each of the following allows you to integrate this chapter into your everyday life. Choose one from this list to complete. a. Interview someone who has been out of work for over a month. Is this...
-
Select three important relationships in your life. These might include your relationships with people at work or school, or with friends and family. For each relationship, rate on a scale ranging...
-
Sketch the graphs of the equations in Problems 31-38. \(y=4^{x}\)
-
A 10-year U.S. Treasury bond with a face value of $10,000 pays a coupon of 5.5% (2.75% of face value every six months). The semiannually compounded interest rate is 5.2% (a six-month discount rate of...
-
Using Gauss-Jordan elimination, invert this matrix ONLY 0 0 0 0 1
-
Why is it not necessary to know absolute half-cell potentials to determine the emf of an electrochemical cell?
-
What is the voltage between the terminals of a battery in which the contents are in chemical equilibrium?
-
By convention, the anode of a battery is where oxidation takes place. Is this true when the battery is charged, discharged, or both?
-
In the balance sheet, the account, Premium on Bonds Payable, is ? Four thousand bonds with a face value of $1,000 each, are sold at 105. The entry to record the issuance is ? 81.Bond interest paid...
-
If the offering price of an open-end fund is $13.20 per share and the fund is sold with a front-end load of 7%, what is its net asset value? (Round your answer to 2 decimal places.) Net asset value
-
explain how the following entries are calculated: Fish Company had the following transactions during the year 2015. IntExp... a. On April 1, Fish rented excess office space in one of its buildings...

Study smarter with the SolutionInn App


