A balloon filled with oxygen gas at 25 C occupies a volume of 2.1 L. Assuming that
Question:
A balloon filled with oxygen gas at 25 °C occupies a volume of 2.1 L. Assuming that the pressure remains constant, what is the volume at 100 °C?
Strategy
Convert the temperatures to kelvins and use Equation 6.2.
Equation 6.2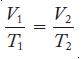
Transcribed Image Text:
V₁ E T₁ V2 T₂ 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
List the data given by the problem Rearrange the equation to p...View the full answer

Answered By

Ajay Negi
Hi, I've completed my degree in engineering (Information Technology) from an NIT. Currently working as a software engineer. Wish to impart quality education to the future generation.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
i) ii) What is the irradiance (in W/m) that falls onto a surface that is positioned perpendicularly to the LED, as shown in the figure below, for a) r = 0 and b) an arbitrary value of r? [6 points]...
-
A balloon filled with 39.1 moles of helium has a volume of 876 L at 0.08C and 1.00 atm pressure. At constant pressure, the temperature of the balloon is increased to 38.08C, causing the balloon to...
-
The small bubbles that form on the bottom of a water pot that is being heated (before boiling) are due to dissolved air coming out of solution. Use Henrys law and the solubilities given to calculate...
-
Suppose \(x\) is a linked-list Node. What is the effect of the following code fragment? \[x \cdot \text { next }=x \cdot \text { next } . \text { next; }\]
-
Use Equation 17-13 to calculate the de Broglie wavelength for an electron of kinetic energy (a) 2.5eV, (b) 250eV, (c) 2.5keV, and (d) 25keV. 1.23 (K in electron volts) 17-13 VK
-
Mountain States Electric Service is an electrical utility company serving several states in the Rocky Mountain region. It is considering replacing some of its equipment at a generating substation and...
-
When should benchmarking be preferred to direct estimates of the magnitudes of benefits? When should direct estimates be preferred? Is it appropriate to use both?
-
Describe what you believe is implied by the term engagement risk. What are the key factors likely considered by Deloitte and other audit firms when assessing engagement risk? How, if at all, are...
-
More generally, evaluate the new Federal Government policies: 1 What are the pros and the cons of the FDIC guarantee of debt? 2. Pros and cons of executive compensation restrictions? 3. Pros and cons...
-
On January 1, 2019, a U.S. company purchased 100% of the outstanding stock of Ventana Grains, a company located in Latz City, New Zealand. Ventana Grains was organized on January 1, 2000. All the...
-
Make a drawing of an open-end manometer measuring the pressure of a sample of a gas that is at 200 torr. Assume the pressure on the open end is 1.00 atm.
-
Th e volume of a sample of nitrogen gas increases from 0.440 L at 27 C to 1.01 L as it is heated to a new temperature. Calculate the new temperature of the nitrogen.
-
The solubility of magnesium fluoride, MgF 2 , in water is 0.015 g/L. What is the solubility (in grams per liter) of magnesium fluoride in 0.17 M sodium fluoride, NaF?
-
Of what crime was Al Capone convicted?
-
Should the SEC take leniency on a company that self-reports accounting improprieties and other frauds?
-
In your opinion, were the ZZZZ Best auditors at fault for not catching this fraud earlier? Why or why not?
-
Given that the ZZZZ Best fraud occurred in 1987, why is society still plagued by financial statement frauds?
-
A man is found shot to death in the front seat of his car. All the windows are closed and the doors are locked; there are no bullet holes anywhere in the car and he did not commit suicide. How was he...
-
A plastics company makes thousands of plastic bottles for another company that manufactures saline solution for users of soft contact lenses. The plastics company randomly inspects a sample of its...
-
Identify the tax issues or problems suggested by the following situations. State each issue as a question. Jennifer did not file a tax return for 2007 because she honestly believed that no tax was...
-
Arrange the following in terms of decreasing bond energy and bond length: O + 2 , O 2 , O - 2 , and O 2- 2 .
-
Images of molecular orbitals for LiH calculated using the minimal basis set are shown here. In these images, the smaller atom is H. The H1s AO has a lower energy than the Li2s AO. The energy of the...
-
What is the electron configuration corresponding to O 2 , O 2 , and O + 2 ? What do you expect the relative order of bond strength to be for these species? Which, if any, have unpaired electrons?
-
During the month of July, the Town of Lynton recorded the following information related to purchases: General government Public safety Public works Culture and recreation Total purchase orders and...
-
Go to the learning style inventories noted for the Module. There are two to choose from (the VARK Questionnaire and the Index of Learning Styles Questionnaire). You can do either or both. Share the...
-
Considering effective communication techniques how would you respond to Mrs . Green.

Study smarter with the SolutionInn App


