Calculate the equilibrium constant from experimental measurements. (a) Nitrogen dioxide dissociates into nitrogen monoxide and oxygen. 2NO(g)
Question:
Calculate the equilibrium constant from experimental measurements.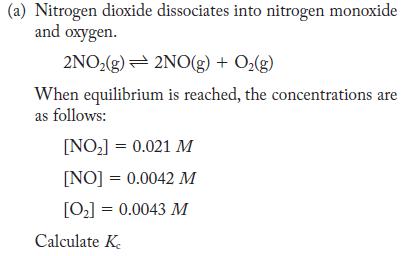
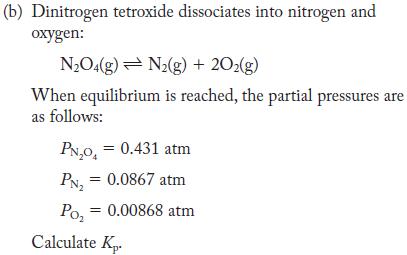
Transcribed Image Text:
(a) Nitrogen dioxide dissociates into nitrogen monoxide and oxygen. 2NO(g) 2NO(g) + O(g) When equilibrium is reached, the concentrations are as follows: [NO] = 0.021 M [NO] = 0.0042 M [0] = 0.0043 M Calculate K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 17...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate the equilibrium constant Kc at 25oC from the free-energy change for the following reaction: See Appendix C for data. Zn(s) +2Ag (a)Zn2 (a) Ag(s)
-
Calculate the equilibrium constant Kc for the following reaction from standard electrode potentials. Fe(s) + Sn**(ag) = Fe* (ag) + Sn*(aq)
-
Determine the vector A-C, given the vectors A and C in the figure. (Figure 1) Figure B (B=26.5) 56.0% (A = 44.0) 28.0 C(C= 31.0) 1 of 1 Determine the magnitude of the vector A - . Express your...
-
Capital budgeting has the same focus as accrual accounting. Do you agree? Explain.
-
In prepared remarks before Congress in mid-2007, Federal Reserve Chairman Ben Bernanke testified: "The principal source of the slowdown in economic growth . has been the substantial correction in the...
-
Briefly describe how development moves from conceptual modeling to logical and physical designs. Provide one example for a non-IS product and one for an IS product.
-
Gunnison Company had the following equivalent units schedule and cost information for its Sewing Department for the month of December: Required: 1. Calculate the unit cost for December, using the...
-
Portland Manufacturing had the following data for the period just ended: Work in process, January 1 Work in process, December 31 Finished goods, January 1 Finished goods, December 31 Direct materials...
-
An equilibrium mixture contains 3.00 mol CO, 2.00 mol Cl 2 , and 9.00 mol COCl 2 in a 50-L reaction flask at 800 K. Calculate the value of the equilibrium constant K c for the reaction at this...
-
Write the expression for the equilibrium constant (K p ) for the following: (a) Cl(g) + HO(g) 2HCl(g) + 0(g) = (b) 2NO(g) NO4(g) (c) 302(g) 203(g) (d) CO(g) CO(g) +0(g) 2
-
Suggest two additional aspects to consider with international focus groups.
-
Alan made 35,000 in taxable income last year. Suppose the income tax rate is 15% for the 9500 first plus 18% for the amount over $8800 How much must Alan pay in income tax for last year?
-
During project evaluation, projected cash flows can change signs from positive to negative and back again. If the signs change more than once, it is best to a. reject the project. b. not bother...
-
Why do you think Ponzi schemes like the one run by Bernie Madoff continue to elude detection? What could be changed to better protect investors against such schemes?
-
Imagine that you are the newly hired CFO for a start-up company that is just beginning to grow. In your role, you must help educate the original leadership staff on the importance of good financial...
-
I dont have the money to afford to pay monthly payments for this website. How am i supposed to complete homework if i dont have free access?
-
The beta of a call option on General Electric is greater than the beta of a share of General Electric. True or false?
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
Consider a skier who coasts up to the top of a hill and then continues down the other side. Draw a qualitative plot of what the skiers speed might look like.
-
The acceleration of an object that falls freely under the action of gravity near the Earths surface is negative and constant. (a) Does the objects instantaneous acceleration equal its average...
-
In the drop zone. Consider a skydiver who jumps from an airplane. Suppose she waits for 1 min before opening her parachute and she lands 4 min after leaving the airplane. Draw qualitative plots of...
-
Suppose that x > 0, y < 0. Using definition of absolute value and splitting into cases, show that | x + y |x y|.
-
A medic's bag contains individually wrapped tongue depressors (each costing 5 cents) and Band-Aids (each costing 10 cents). If the materials are worth $15.80 and there are 180 individual packages in...
-
(1 point) If -4 -1 4 -3 -4 3 5 4 1 1 determine the following entries: a14 = -3 a33 = -1 a21 = 3determi

Study smarter with the SolutionInn App


