Calculate the pH in the titration of 20.00 mL of 0.125 M HCl after the addition of
Question:
Calculate the pH in the titration of 20.00 mL of 0.125 M HCl after the addition of
(a) 0,
(b) 2.00,
(c) 10.00, and
(d) 20.00 mL of 0.250 M NaOH.
Strategy
First, calculate the amounts (mmol) of acid and base. The analyte and the titrant (acid and base) react with each other, and this reaction goes to completion. Use the stoichiometry of the reaction to fill out the sRfc table after each addition of titrant. We need to determine which species remain after the titration. The logic flow diagram is shown below.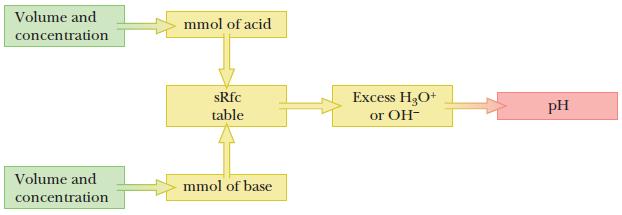
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





