Calculate the standard free energy change for the reaction Strategy Use Table 18.1 to determine the cell
Question:
Calculate the standard free energy change for the reaction![]()
Strategy Use Table 18.1 to determine the cell potential; then use Equation 18.2 to calculate the ΔG°. You will also have to determine the number of moles of electrons, n, that are transferred in this reaction.
Equation 18.2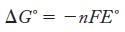
Table 18.1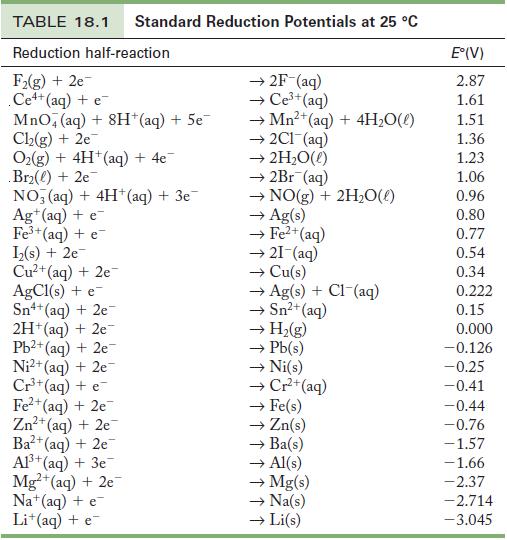
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





