Consider the decomposition of nitrogen dioxide. At 650 K , the rate constant is 1.66 L/mol
Question:
Consider the decomposition of nitrogen dioxide.![]()
At 650 K , the rate constant is 1.66 L/mol · s ; at 700 K , it is 7.39 L/mol · s . Use these rate constants to determine the activation energy.
Strategy
Equation 13.11 relates two rate constants and two temperatures. Let subscript 1 in Equation 13.11 represent the lower temperature and corresponding rate; let subscript 2 represent the higher temperature data. Because it is conventional to express activation energy in J/mol, we will use 8.314 J/mol · K for R.
Equation 13.11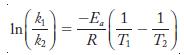
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





