If a volume of 32.45 mL HCl is used to completely neutralize 2.050 g Na 2 CO
Question:
If a volume of 32.45 mL HCl is used to completely neutralize 2.050 g Na2CO3 according to the following equation, what is the molarity of the HCl?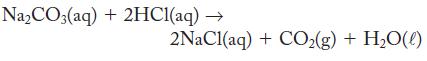
Transcribed Image Text:
Na₂CO3(aq) + 2HC1(aq) → 2NaCl(aq) + CO₂(g) + H₂O(0)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine the molarity of the HCl solution we need to use the volume and mass data pro...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
134+ Reviews
426+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
If 25.30 mL of 0.277 M HCl is used to titrate 10.0 mL of aqueous ammonia to a methyl red endpoint, what is the molarity of the ammonia? When we write aqueous ammonia as NH 4 OH, the balanced equation...
-
The volume of a sample of pure HCl gas was 189 mL at 25C and 108 mmHg. It was completely dissolved in about 60 mL of water and titrated with an NaOH solution; 15.7 mL of the NaOH solution were...
-
The adjusted trial balance for Ray Corporation at July 31, 2017, the corporation's fiscal year end, contained the following: Of the lease liability amount, $16,250 is due within the next year. Total...
-
We consider the random process St, which plays a fundamental role in BIack-Scholes analyses: St = S0e[1+Wt] Where Wt is a Wiener process with W0 = 0, is a trend factor, and (Wt Ws) N(0, (I s)),...
-
Use Figure 3.22 to evaluate the derivative. 80 0 # f(x) 80 $6 0 Figure 3.22 80
-
The very long cylindrical solenoid of Figure P29.27 has a radius of \(0.50 \mathrm{~m}\) and 1000 windings per meter along its length. A circular conducting loop of radius \(1.0 \mathrm{~m}\)...
-
A job order cost sheet for Ryan Company is shown below. Instructions (a) On the basis of the foregoing data, answer the following questions. (1) What was the balance in Work in Process Inventory on...
-
Part A A string that is 9.6 m long is tied between two posts and plucked. The string produces a wave that has a frequency of 320 Hz and travels with a speed of 192 m/s. How many full wavelengths of...
-
Air is compressed in an isentropic compressor from 15 psia and 70°F to 200 psia. Determine the outlet temperature and the work consumed by this compressor per unit mass of air. 200 psia Air...
-
What is the molar concentration of a solution of HNO 3 if 50.00 mL react completely with 22.40 mL of a 0.0229 M solution of Sr(OH) 2 ?
-
Write the overall equation (including the physical states), the complete ionic equation, and the net ionic equation for the reaction of aqueous solutions of sodium hydroxide and magnesium chloride....
-
What minimum classifications must be used to report net position in the government-wide Statement of Net Position? In the proprietary funds Statement of Net Position?
-
Discuss the following statement: Non-verbal communication is more effective than verbal communication.
-
How can an organization create a positive climate for voice and what impact does it have on employees?
-
Discuss the relationship between size and structure.
-
Define flex time and telecommuting. How does each affect the quality of working life?
-
What is job crafting and what are the different ways that employees can engage in job crafting? How is job crafting different from other approaches to job design?
-
A university Web server has five main components, each with the same reliability. All five components must work for the server to function as intended. If the university wants to have a 95 percent...
-
List four items of financial information you consider to be important to a manager of a business that has been operating for a year.
-
Predict the products for each of the following: a. b. c. d. 1) Hg(OAc), 2) NABH, 1) Hg(OAc), e 2) NaBH,
-
Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds. Each compound has a Br and one other substituent that we did not...
-
Benzene was treated with (R)-2-chlorobutane in the presence of aluminum trichloride, and the resulting product mixture was found to be optically inactive. (a) What products are expected, assuming...
-
On July 22, 2022, the Free Tress, a Libra oil tanker collided with a container ship, the Ocean Breeze, in the Malacca Straits, the Free Tress was laden with crude oil. Following the collision...
-
K What is the minimum cost of crashing the following project that Roger Solano manages at Slippery Rock University by 4 days? Total Cost $1,100 Normal Crash Normal Activity Time (days) Time (days)...
-
What type of qualitative and quantitative methods of data can be used to collect this data?

Study smarter with the SolutionInn App


