Nitramide decomposes to water and dinitrogen monoxide. This reaction was studied by J. N. Brnsted in
Question:
Nitramide decomposes to water and dinitrogen monoxide.![]()
This reaction was studied by J. N. Brønsted in 1924 as part of research into the fundamental nature of acids and bases. If 1.00 L of 0.440 M nitramide is placed in a reactor at 20 °C, the following results are expected. (The experiment was actually performed to measure the effect of malate ion on the rate of reaction.)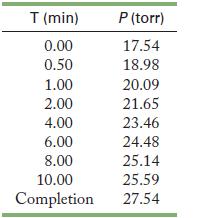 What is the rate law for this reaction?
What is the rate law for this reaction?
Graph (final pressure = current pressure), rather than the current pressure, as a function of time to see the data. If this is not a straight line, you can graph ln(final pressure - current pressure) and 1/(final pressure - current pressure) to determine the order of the reaction. Scientists frequently transform their data to a familiar form. Th ese operations make it easier to interpret the data.
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





