Picric acid, or 2,4,6-trinitrophenol, has the following structure: Not only is it an acid, it is also
Question:
Picric acid, or 2,4,6-trinitrophenol, has the following structure: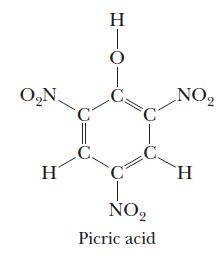

Not only is it an acid, it is also an explosive.
(a) Based on what you know of oxyacids, which hydrogen ionizes from the picric acid molecule?
(b) Picric acid has a Ka of 0.380. What is the pH of a 0.100 M solution of picric acid? You will have to use the quadratic formula.
(c) When picric acid explodes anaerobically (that is, without oxygen), it forms carbon monoxide, water, nitrogen, and solid carbon. Write the balanced chemical equation for the decomposition of picric acid.
(d) Would substitution of the nitro (NO2) groups in the molecule with amino (NH2) likely increase or decrease the Ka of the compound? Explain your answer.
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





