Th e formation of hydrogen chloride is exothermic: What are the values of for H(g) + Ch(g)
Question:
Th e formation of hydrogen chloride is exothermic:![]()
What are the values of![]() for
for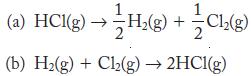
Transcribed Image Text:
H₂(g) + Ch₂(g) - Cl₂(g) → HCl(g) ΔΗ = −92.3 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To determine the values of H for the given reactions we can use Hesss l...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A $940 face value bond with a quoted price of 90 is selling for
-
Heating acetone with an excess of phenol in the presence of hydrogen chloride is the basis for an industrial process used in the manufacture of a compound called bisphenol A. (Bisphenol A, about...
-
5) Using the figure below, what is the magnitude of the resultant force for the uniform distributed load shown below? |-- 40 N/m 50 N 100 N/m 2m 1m 4 m Grade
-
During 2018, Susan incurred and paid the following expenses for Beth (her daughter), Ed (her father), and herself: Surgery for...
-
Use the method of lines with a five-point, biased, upwind finite-difference approximation and a stiff integrator to perform PSA cycle calculations that approach the cyclic steady state for the data...
-
What are the hypotheses and conclusion of the Mean Value Theorem? What physical interpretations might the theorem have?
-
Why is leasing versus buying an unfair comparison?
-
Warner Company started business on January 1, 2011. The following transactions and events occurred in 2011 and 2012. For simplicity, information for sales, inventory purchases, collections on...
-
A ( n ) _ _ _ _ _ _ _ _ _ _ contains information about a given person, product, or event. Attribute Column Field Record
-
On December 1, Year 1, John and Patty Driver formed a corporation called Susquehanna Equipment Rentals. The new corporation was able to begin operations immediately by purchasing the assets and...
-
Methane, CH 4 (g), and octane, , are important components of the widely used fossil fuels. Th e enthalpy change for combustion of 1 mol methane is -890 kJ, and that for 1 mol octane is -5466 kJ....
-
Draw an energy-level diagram for an endothermic reaction of the following type: reactants products
-
Of the many species of oak trees in the United States, 28 grow on the Atlantic Coast and 11 grow in California. The back-to-back stemplot displays data on the average volume of acorns (in cubic...
-
Mens rea, Latin for guilty mind, is the second critical component of criminal liability. General intent crimes are those offenses that contain no specific mens rea component. Specific intent crimes...
-
Actus reus is Latin for evil act. There cannot be a crime without a criminal act. In general, a person cannot be guilty of a crime unless that person commits a voluntary act. An omission is a...
-
The general part of the criminal law is general because it is not unique to any one crime. For example, underlying every crime is a prohibited act or omission. The special part of the criminal law...
-
Causation, the requirement that the defendant is responsible for the harm, applies only to result crimes. Factual causation (or cause in fact) requires that there can be no criminal liability for a...
-
Kathy and Mark set off in late fall on a romantic camping trip on the Appalachian Trail. Mark, however, was not very skilled at camping and forgot to pack any type of tent or sleeping bag. As night...
-
Many firms have devised defenses that make it more difficult or costly for other firms to take them over. How might such defenses affect the firms agency problems? Are managers of firms with...
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
Express the following complex numbers in the form a + ib. a. 2e 3i /2 b. 43 e i/4 c. e i d. 5 / 1+ 2 e i/4
-
One source emits spherical waves and another emits plane waves. For which source does the intensity measured by a detector of fixed size fall off more rapidly with distance? Why?
-
What is the relationship between evaluating an integral and graphing the integrand?
-
Replacement of a buried isoleucine residue with alanine increases the observed entropy of unfolding for a protein. Explain this observation. The diagram on the right may be helpful, the x- axis is...
-
A company that makes shopping carts for supermarkets and other stores recently purchased some new equipment that reduces the labor content of the jobs needed to produce the shopping carts. Prior to...
-
Jimmy invests $15,000 in an account that pays 6.07% compounded quarterly. How long (in years and months) will it take for his investment to reach $22,000? years and months (Round the answer for...

Study smarter with the SolutionInn App


